Immune Netw.
2016 Dec;16(6):358-365. 10.4110/in.2016.16.6.358.
Induction of Indoleamine 2,3-dioxygenase by Pre-treatment with Poly(I:C) May Enhance the Efficacy of MSC Treatment in DSS-induced Colitis
- Affiliations
-
- 1Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. ckmin@catholic.ac.kr
- 2Department of Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2362800
- DOI: http://doi.org/10.4110/in.2016.16.6.358
Abstract
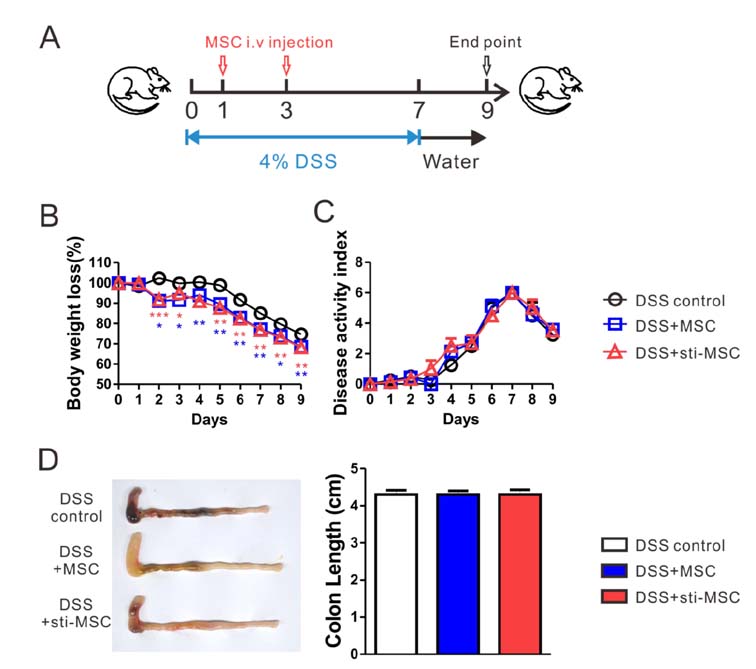
- Mesenchymal stem cells (MSCs) have been used experimentally for treating inflammatory disorders, partly owing to their immunosuppressive properties. The goal of the study was to determine whether TLR ligands can enhance the therapeutic efficacy of bone marrow-derived MSCs for the treatment of inflammatory bowel disease. Mice (C57BL6) were administered with 4% dextran sulfate sodium (DSS) in drinking water for 7 days and injected with MSCs on days 1 and 3 following DSS ingestion. Our results demonstrated that among various TLR ligands, MSCs treated with polyinosinic-polycytidylic acid [poly(I:C)], which is a TLR3 ligand, more profoundly induced IDO, which is a therapeutically relevant immunosuppressive factor, without any observable phenotype change in vitro. The poly(I:C)-treated MSCs attenuated the pathologic severity of DSS-induced murine colitis when injected i.p. but not i.v. In summary, preconditioning MSCs with poly(I:C) might improve their efficacy in treating DSS-induced colitis, and this effect at least partly depends on the enhancement of their immunosuppressive activity through increasing their production of IDO.
Keyword
MeSH Terms
-
Animals
Colitis*
Dextran Sulfate
Drinking Water
Eating
In Vitro Techniques
Indoleamine-Pyrrole 2,3,-Dioxygenase*
Inflammatory Bowel Diseases
Ligands
Mesenchymal Stromal Cells
Mice
Phenotype
Poly I-C
Toll-Like Receptors
Dextran Sulfate
Drinking Water
Indoleamine-Pyrrole 2,3,-Dioxygenase
Ligands
Poly I-C
Toll-Like Receptors
Figure
Reference
-
1. Tsagias N, Koliakos I, Karagiannis V, Eleftheriadou M, Koliakos GG. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus Med. 2011; 21:253–261.
Article2. Liang L, Dong C, Chen X, Fang Z, Xu J, Liu M, Zhang X, Gu DS, Wang D, Du W, Zhu D, Han ZC. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011; 20:1395–1408.
Article3. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007; 25:2739–2749.
Article4. DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010; 2010:865601.
Article5. English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007; 110:91–100.
Article6. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009; 156:149–160.
Article7. Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010; 88:795–806.
Article8. Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007; 109:228–234.
Article9. Nishiyama Y, Kataoka T, Yamato K, Taguchi T, Yamaoka K. Suppression of dextran sulfate sodium-induced colitis in mice by radon inhalation. Mediators Inflamm. 2012; 2012:239617.
Article10. Lim JY, Kim BG, Moon SK, Min CK. Enhancement of graft-versus-leukemia effects by mesenchymal stem cells in mixed chimerisim after a murine non-myeloablative hematopoietic stem cell transplantation. Korean J Hematol. 2008; 43:219–231.
Article11. Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Caprilli R. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999; 44:1458–1475.12. Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipor D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007; 109:1422–1432.
Article13. Mastri M, Shah Z, McLaughlin T, Greene CJ, Baum L, Suzuki G, Lee T. Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency. Am J Physiol Cell Physiol. 2012; 303:C1021–C1033.
Article14. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011; 9:11–15.
Article15. Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Koppel A, Tolosa E, Hoberg M, Anderl J, Aicher WK, Weller M, Wick W, Platten M. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase. R Stem Cells. 2009; 27:909–919.
Article16. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009; 54:2277–2286.
Article17. Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008; 26:279–289.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of placental indoleamine 2,3-dioxygenase in human pregnancy
- The Therapeutic Efficacy of Tonsil-derived Mesenchymal Stem Cells in Dextran Sulfate Sodium-induced Acute Murine Colitis Model
- The tryptophan utilization concept in pregnancy
- Anti-colitis efficacy of oxyresveratrol isolated from mulberry twig in dextran sulfate sodium-induced mouse colitis
- Long-Term Effects of Bone Marrow-Derived Mesenchymal Stem Cells in Dextran Sulfate Sodium-Induced Murine Chronic Colitis