Obstet Gynecol Sci.
2014 Jul;57(4):249-259. 10.5468/ogs.2014.57.4.249.
The tryptophan utilization concept in pregnancy
- Affiliations
-
- 1School of Health Sciences, Cardiff Metropolitan University, Wales, UK. ABadawy@cardiffmet.ac.uk
- KMID: 1841535
- DOI: http://doi.org/10.5468/ogs.2014.57.4.249
Abstract
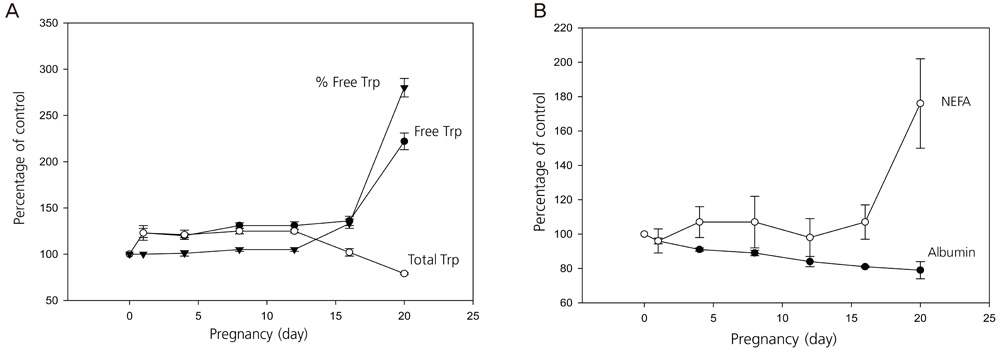
- The decrease in maternal plasma total (free + albumin-bound) tryptophan (Trp) during the third pregnancy trimester is attributed to induction of indoleamine 2,3-dioxygenase (IDO). When measured, free [Trp] is increased because of albumin depletion and non-esterified fatty acid elevation. The Trp depletion concept in pregnancy is therefore not supported because of incorrect interpretation of changes in Trp disposition and also for not addressing mouse strain differences in Trp-related responses and potential inhibition of Trp transport by the IDO inhibitor 1-methyl tryptophan. Application of the Trp utilization concept in pregnancy offers several physiological advantages favoring fetal development and successful outcome, namely provision of Trp for fetal protein synthesis and growth, serotonin for signaling pathways, kynurenic acid for neuroprotection, quinolinic acid for NAD+ synthesis, and other kynurenines for suppression of T cell responses. An excessive increase in Trp availability could compromise pregnancy by undermining T cell suppression, e.g., in pre-eclampsia.
MeSH Terms
Figure
Reference
-
1. Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983; 6:101–197.2. Badawy AA. Tryptophan metabolism in alcoholism. Nutr Res Rev. 2002; 15:123–152.3. Badawy AA. Effects of pregnancy on tryptophan metabolism and disposition in the rat. Biochem J. 1988; 255:369–372.4. Badawy AA, Evans M. Animal liver tryptophan pyrrolases: absence of apoenzyme and of hormonal induction mechanism from species sensitive to tryptophan toxicity. Biochem J. 1976; 158:79–88.5. Pfefferkorn ER, Rebhun S, Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J Interferon Res. 1986; 6:267–279.6. Werner ER, Bitterlich G, Fuchs D, Hausen A, Reibnegger G, Szabo G, et al. Human macrophages degrade tryptophan upon induction by interferon-gamma. Life Sci. 1987; 41:273–280.7. Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents on induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochem Biophys Res Commun. 1987; 144:1147–1153.8. Badawy AA. Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J Psychopharmacol. 2013; 27:878–893.9. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991; 5:2516–2522.10. Daubener W, MacKenzie CR. IFN-gamma activated indoleamine 2,3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv Exp Med Biol. 1999; 467:517–524.11. Yamazaki F, Kuroiwa T, Takikawa O, Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem J. 1985; 230:635–638.12. Kudo Y. The role of placental indoleamine 2,3-dioxygenase in human pregnancy. Obstet Gynecol Sci. 2013; 56:209–216.13. Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998; 281:1191–1193.14. De Antoni A, Allegri G, Costa C, Vanzan S, Bertolin A, Carretti N, et al. Total and free tryptophan levels in serum of newborn infants: relationships with the serotonin and nicotinic acid pathways. Acta Vitaminol Enzymol. 1980; 2:17–20.15. Handley SL, Dunn TL, Waldron G, Baker JM. Tryptophan, cortisol and puerperal mood. Br J Psychiatry. 1980; 136:498–508.16. Morita I, Kawamoto M, Yoshida H. Difference in the concentration of tryptophan metabolites between maternal and umbilical foetal blood. J Chromatogr. 1992; 576:334–339.17. Schrocksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H, Fuchs D. Decreased plasma tryptophan in pregnancy. Obstet Gynecol. 1996; 88:47–50.18. Abou-Saleh MT, Ghubash R, Karim L, Krymski M, Ibrahim A. Postpartum mood changes and plasma amino-acids. Curr Psychiatry. 1998; 5:314–319.19. Maes M, Ombelet W, Verkerk R, Bosmans E, Scharpe S. Effects of pregnancy and delivery on the availability of plasma tryptophan to the brain: relationships to delivery-induced immune activation and early post-partum anxiety and depression. Psychol Med. 2001; 31:847–858.20. Wachter H, Fuchs D, Hausen A, Reibnegger G, Werner ER. Neopterin as marker for activation of cellular immunity: immunologic basis and clinical application. Adv Clin Chem. 1989; 27:81–141.21. Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Yim JJ, et al. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem. 1990; 265:3189–3192.22. Badawy AA. Plasma free tryptophan revisited: what you need to know and do before measuring it. J Psychopharmacol. 2010; 24:809–815.23. Sherlock S. The liver in pregnancy. In : Sherlock S, editor. Diseases of the liver and biliary system. Oxford: Blackwell;1981. p. 400–405.24. Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000; 54:Suppl 1. S47–S51.25. Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009; 114:1326–1331.26. Laron Z, Mannheimer S, Nitzan M, Goldman J. Growth hormone, glucose, and free fatty acid levels in mother and infant in normal, diabetic and toxaemic pregnancies. Arch Dis Child. 1967; 42:24–28.27. Chen X, Scholl TO. Association of elevated free fatty acids during late pregnancy with preterm delivery. Obstet Gynecol. 2008; 112(2 Pt 1):297–303.28. El Beltagy NS, Sadek SS, Zidan MA, Abd El Naby RE. Can serum free fatty acids assessment predict severe preeclampsia? Alexandria J Med. 2011; 47:277–281.29. Altman K, Greengard O. Correlation of kynurenine excretion with liver tryptophan pyrrolase levels in disease and after hydrocortisone induction. J Clin Invest. 1966; 45:1527–1534.30. World Health Organisation. Protein and amino acid requirements in human nutrition: report of a joint WHO/FAO/UNU expert consultation. Geneva: World Health Organisation;2007.31. Moe AJ. Placental amino acid transport. Am J Physiol. 1995; 268(6 Pt 1):C1321–C1331.32. Carretti N, Bertazzo A, Comai S, Costa CV, Allegri G, Petraglia F. Serum tryptophan and 5-hydroxytryptophan at birth and during post-partum days. Adv Exp Med Biol. 2003; 527:757–760.33. Moniz CF, Nicolaides KH, Bamforth FJ, Rodeck CH. Normal reference ranges for biochemical substances relating to renal, hepatic, and bone function in fetal and maternal plasma throughout pregnancy. J Clin Pathol. 1985; 38:468–472.34. Tsuji A, Nakata C, Sano M, Fukuwatari T, Shibata K. L-tryptophan metabolism in pregnant mice fed a high L-tryptophan diet and the effect on maternal, placental, and fetal growth. Int J Tryptophan Res. 2013; 6:21–33.35. Hiratsuka C, Fukuwatari T, Sano M, Saito K, Sasaki S, Shibata K. Supplementing healthy women with up to 5.0 g/d of L-tryptophan has no adverse effects. J Nutr. 2013; 143:859–866.36. Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003; 81:247–265.37. Mellor AL, Munn D, Chandler P, Keskin D, Johnson T, Marshall B, et al. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003; 527:27–35.38. Schrocksnadel K, Widner B, Bergant A, Neurauter G, Schrocksnadel H, Fuchs D. Tryptophan degradation during and after gestation. Adv Exp Med Biol. 2003; 527:77–83.39. Werner ER, Fuchs D, Hausen A, Jaeger H, Reibnegger G, Werner-Felmayer G, et al. Tryptophan degradation in patients infected by human immunodeficiency virus. Biol Chem Hoppe Seyler. 1988; 369:337–340.40. Fuchs D, Forsman A, Hagberg L, Larsson M, Norkrans G, Reibnegger G, et al. Immune activation and decreased tryptophan in patients with HIV-1 infection. J Interferon Res. 1990; 10:599–603.41. Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem. 1986; 261:3648–3653.42. Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino Acids. 2011; 41:1173–1183.43. Kudo Y, Boyd CA. The role of L-tryptophan transport in L-tryptophan degradation by indoleamine 2,3-dioxygenase in human placental explants. J Physiol. 2001; 531(Pt 2):417–423.44. Vumma R, Johansson J, Lewander T, Venizelos N. Tryptophan transport in human fibroblast cells-a functional characterization. Int J Tryptophan Res. 2011; 4:19–27.45. Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991; 291:326–333.46. Iizuka H, Sugano H, Yajima T. Fluorometric determination of L-kynurenine with glycolaldehyde by high performance liquid chromatography. Adv Exp Med Biol. 1996; 398:749–753.47. Garber K. Evading immunity: new enzyme implicated in cancer. J Natl Cancer Inst. 2012; 104:349–352.48. Kiank C, Zeden JP, Drude S, Domanska G, Fusch G, Otten W, et al. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS One. 2010; 5:e11825.49. Monroe CB. Induction of tryptophan oxygenase and tyrosine aminotransferase in mice. Am J Physiol. 1968; 214:1410–1414.50. Badawy AA, Evans M. The role of free serum tryptophan in the biphasic effect of acute ethanol administration on the concentrations of rat brain tryptophan, 5-hydroxytryptamine and 5-hydroxyindol-3-ylacetic acid. Biochem J. 1976; 160:315–324.51. Badawy AA, Morgan CJ, Lane J, Dhaliwal K, Bradley DM. Liver tryptophan pyrrolase. A major determinant of the lower brain 5-hydroxytryptamine concentration in alcohol-preferring C57BL mice. Biochem J. 1989; 264:597–599.52. Bano S. Tryptophan metabolism in relation to mental illness [dissertation]. Cardiff: Cardiff University;1997.53. O'Connor MA, Green WR. The role of indoleamine 2,3-dioxygenase in LP-BPM5 murine retroviral disease progression. Virol J. 2013; 10:154.54. Carrera-Silva EA, Cano RC, Guinazu N, Aoki MP, Pellegrini A, Gea S. TLR2, TLR4 and TLR9 are differentially modulated in liver lethally injured from BALB/c and C57BL/6 mice during Trypanosoma cruzi acute infection. Mol Immunol. 2008; 45:3580–3588.55. Soudi S, Zavaran-Hosseini A, Muhammad Hassan Z, Soleimani M, Jamshidi Adegani F, Hashemi SM. Comparative study of the effect of LPS on the function of BALB/c and C57BL/6 peritoneal macrophages. Cell J. 2013; 15:45–54.56. Bernardi F, Guolo F, Bortolin T, Petronilho F, Dal-Pizzol F. Oxidative stress and inflammatory markers in normal pregnancy and preeclampsia. J Obstet Gynaecol Res. 2008; 34:948–951.57. Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998; 179:80–86.58. Szarka A, Rigo J Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010; 11:59.59. Borzychowski AM, Croy BA, Chan WL, Redman CW, Sargent IL. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol. 2005; 35:3054–3063.60. Cayci T, Akgul EO, Kurt YG, Aydin I, Alacam H, Ozkan E, et al. Cord blood and maternal serum neopterin concentrations in patients with pre-eclampsia. Clin Chem Lab Med. 2010; 48:1127–1131.61. Nilsen RM, Bjorke-Monsen AL, Midttun O, Nygard O, Pedersen ER, Ulvik A, et al. Maternal tryptophan and kynurenine pathway metabolites and risk of preeclampsia. Obstet Gynecol. 2012; 119:1243–1250.62. Taniguchi K, Okatani Y, Sagara Y. Serotonin metabolism in the fetus in preeclampsia. Asia Oceania J Obstet Gynaecol. 1994; 20:77–86.63. Oian P, Kjeldsen SE, Eide I, Maltau JM. Increased arterial catecholamines in pre-eclampsia. Acta Obstet Gynecol Scand. 1986; 65:613–617.64. Manyonda IT, Slater DM, Fenske C, Hole D, Choy MY, Wilson C. A role for noradrenaline in pre-eclampsia: towards a unifying hypothesis for the pathophysiology. Br J Obstet Gynaecol. 1998; 105:641–648.65. Endresen MJ, Lorentzen B, Henriksen T. Increased lipolytic activity of sera from pre-eclamptic women due to the presence of a lysophospholipase. Scand J Clin Lab Invest. 1993; 53:733–739.66. Badawy AA, Evans M. Regulation of rat liver tryptophan pyrrolase by its cofactor haem: experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975; 150:511–520.67. Gal EM, Young RB, Sherman AD. Tryptophan loading: consequent effects on the synthesis of kynurenine and 5-hydroxyindoles in rat brain. J Neurochem. 1978; 31:237–244.68. Evans RW, Powers RW, Ness RB, Cropcho LJ, Daftary AR, Harger GF, et al. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction. 2003; 125:785–790.69. Moiseiwitsch JR. The role of serotonin and neurotransmitters during craniofacial development. Crit Rev Oral Biol Med. 2000; 11:230–239.70. Cordeaux Y, Pasupathy D, Bacon J, Charnock-Jones DS, Smith GC. Characterization of serotonin receptors in pregnant human myometrium. J Pharmacol Exp Ther. 2009; 328:682–691.71. Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007; 104:329–334.72. Bonnin A, Levitt P. Placental source for 5-HT that tunes fetal brain development. Neuropsychopharmacology. 2012; 37:299–300.73. Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993; 45:309–379.74. Von Bubnoff D, Matz H, Frahnert C, Rao ML, Hanau D, de la Salle H, et al. FcepsilonRI induces the tryptophan degradation pathway involved in regulating T cell responses. J Immunol. 2002; 169:1810–1816.75. Badawy AA, Morgan CJ. Rapid isocratic liquid chromatographic separation and quantification of tryptophan and six kynurenine metabolites in biological samples with ultraviolet and fluorimetric detection. Int J Tryptophan Res. 2010; 3:175–186.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Eosinophilia-Myalgia Syndrome not Associated with L-tryptophan: A case report
- The role of placental indoleamine 2,3-dioxygenase in human pregnancy
- Acute Tryptophan Depletion and Functional Brain Imaging in Irritable Bowel Syndrome (Gut 2011;60:1196-1203)
- The Effect of Treatment with Tryptophan and/or Imipramine on the Serotonergic Immunoreactivity in Raphe Nucleus of Midbrain of the Rats
- Eosinophilia-Myalgia Syndrome not Associated with the Ingestion of Nutritional Supplements