Korean J Pain.
2016 Oct;29(4):229-238. 10.3344/kjp.2016.29.4.229.
Lipid emulsion inhibits vasodilation induced by a toxic dose of bupivacaine by suppressing bupivacaine-induced PKC and CPI-17 dephosphorylation but has no effect on vasodilation induced by a toxic dose of mepivacaine
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Gyeongsang National University School of Medicine and Gyeongsang National University Hospital, Jinju, Korea. jtsohn@nongae.gsnu.ac.kr
- 2Department of Physiology, Institute for Clinical and Translational Research, Catholic Kwangdong University College of Medicine, Gangneung, Korea.
- 3Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, Jinju, Korea.
- 4Department of Anesthesiology and Pain Medicine, Pusan National University Hospital, Biomed Research Institute, Pusan National University School of Medicine, Busan, Korea.
- 5Institute of Health Sciences, Gyeongsang National University, Jinju, Korea.
- KMID: 2354215
- DOI: http://doi.org/10.3344/kjp.2016.29.4.229
Abstract
- BACKGROUND
The goal of this in vitro study was to investigate the effect of lipid emulsion on vasodilation caused by toxic doses of bupivacaine and mepivacaine during contraction induced by a protein kinase C (PKC) activator, phorbol 12,13-dibutyrate (PDBu), in an isolated endothelium-denuded rat aorta.
METHODS
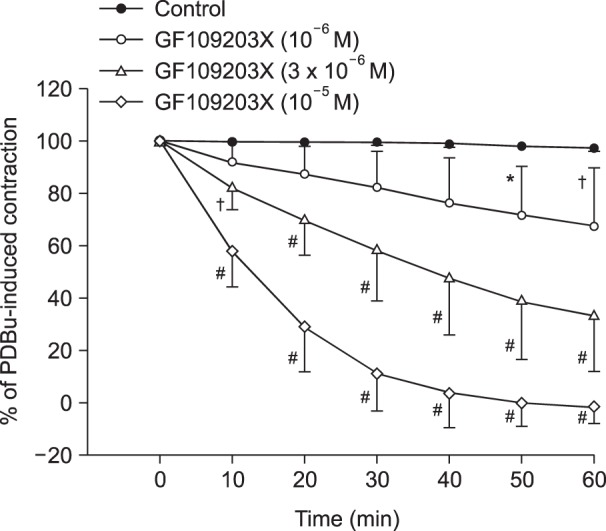
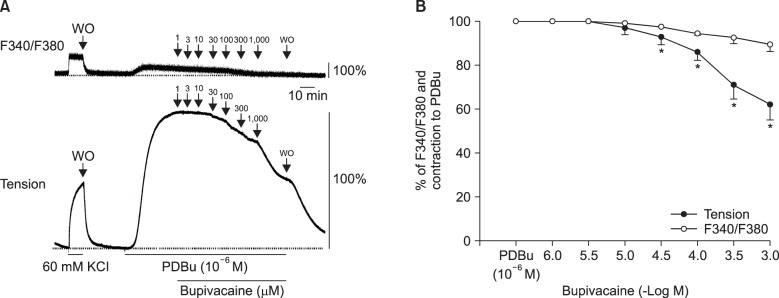
The effects of lipid emulsion on the dose-response curves induced by bupivacaine or mepivacaine in an isolated aorta precontracted with PDBu were assessed. In addition, the effects of bupivacaine on the increased intracellular calcium concentration ([Ca²âº]áµ¢) and contraction induced by PDBu were investigated using fura-2 loaded aortic strips. Further, the effects of bupivacaine, the PKC inhibitor GF109203X and lipid emulsion, alone or in combination, on PDBu-induced PKC and phosphorylation-dependent inhibitory protein of myosin phosphatase (CPI-17) phosphorylation in rat aortic vascular smooth muscle cells (VSMCs) was examined by western blotting.
RESULTS
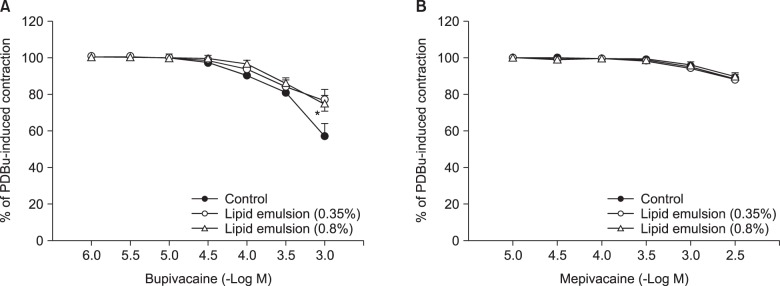
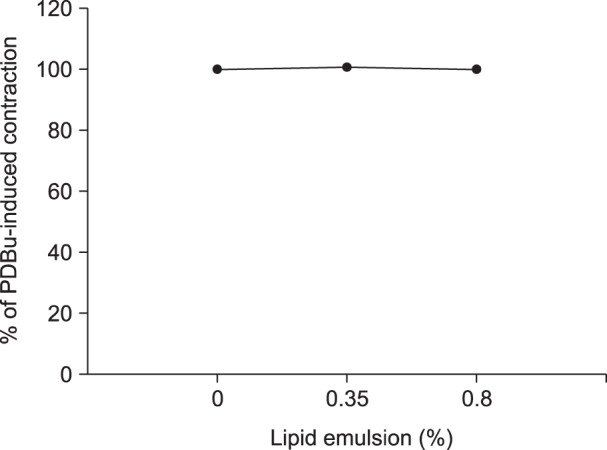
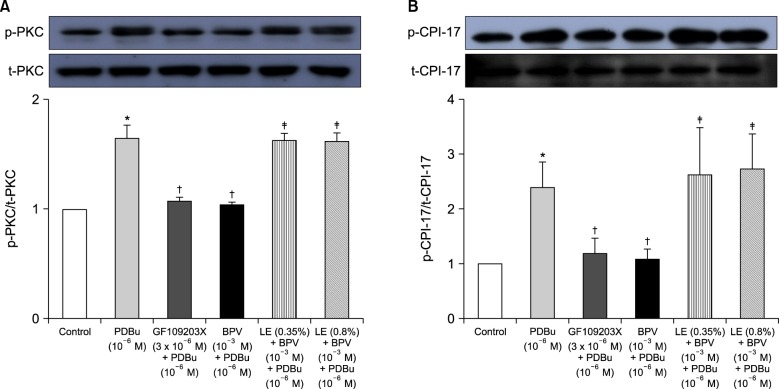
Lipid emulsion attenuated the vasodilation induced by bupivacaine, whereas it had no effect on that induced by mepivacaine. Lipid emulsion had no effect on PDBu-induced contraction. The magnitude of bupivacaine-induced vasodilation was higher than that of the bupivacaine-induced decrease in [Ca²âº]áµ¢. PDBu promoted PKC and CPI-17 phosphorylation in aortic VSMCs. Bupivacaine and GF109203X attenuated PDBu-induced PKC and CPI-17 phosphorylation, whereas lipid emulsion attenuated bupivacaine-mediated inhibition of PDBu-induced PKC and CPI-17 phosphorylation.
CONCLUSIONS
These results suggest that lipid emulsion attenuates the vasodilation induced by a toxic dose of bupivacaine via inhibition of bupivacaine-induced PKC and CPI-17 dephosphorylation. This lipid emulsion-mediated inhibition of vasodilation may be partly associated with the lipid solubility of local anesthetics.
Keyword
MeSH Terms
-
Anesthetics, Local
Animals
Aorta
Blotting, Western
Bupivacaine*
Calcium
Fura-2
In Vitro Techniques
Mepivacaine*
Muscle, Smooth, Vascular
Myosin-Light-Chain Phosphatase
Phorbol 12,13-Dibutyrate
Phosphorylation
Protein Kinase C
Rats
Solubility
Vasodilation*
Anesthetics, Local
Bupivacaine
Calcium
Fura-2
Mepivacaine
Myosin-Light-Chain Phosphatase
Phorbol 12,13-Dibutyrate
Protein Kinase C
Figure
Reference
-
1. Weinberg GL. Lipid emulsion infusion: resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012; 117:180–187. PMID: 22627464.2. Cao D, Heard K, Foran M, Koyfman A. Intravenous lipid emulsion in the emergency department: a systematic review of recent literature. J Emerg Med. 2015; 48:387–397. PMID: 25534900.
Article3. Ok SH, Han JY, Lee SH, Shin IW, Lee HK, Chung YK, et al. Lipid emulsion-mediated reversal of toxic-dose aminoamide local anesthetic-induced vasodilation in isolated rat aorta. Korean J Anesthesiol. 2013; 64:353–359. PMID: 23646246.
Article4. Ok SH, Han JY, Sung HJ, Yang SM, Park J, Kwon SC, et al. Ropivacaine-induced contraction is attenuated by both endothelial nitric oxide and voltage-dependent potassium channels in isolated rat aortae. Biomed Res Int. 2013; 2013:565271. PMID: 24350275.
Article5. Ok SH, Kwon SC, Kang S, Choi MJ, Sohn JT. Mepivacaine-induced intracellular calcium increase appears to be mediated primarily by calcium influx in rat aorta without endothelium. Korean J Anesthesiol. 2014; 67:404–411. PMID: 25558341.
Article6. Baik JS, Sohn JT, Ok SH, Kim JG, Sung HJ, Park SS, et al. Levobupivacaine-induced contraction of isolated rat aorta is calcium dependent. Can J Physiol Pharmacol. 2011; 89:467–476. PMID: 21812525.
Article7. Ok SH, Sohn JT, Baik JS, Kim JG, Park SS, Sung HJ, et al. Lipid emulsion reverses Levobupivacaine-induced responses in isolated rat aortic vessels. Anesthesiology. 2011; 114:293–301. PMID: 21239969.
Article8. Mulroy MF. Systemic toxicity and cardiotoxicity from local anesthetics: incidence and preventive measures. Reg Anesth Pain Med. 2002; 27:556–561. PMID: 12430104.
Article9. Akata T. General anesthetics and vascular smooth muscle: direct actions of general anesthetics on cellular mechanisms regulating vascular tone. Anesthesiology. 2007; 106:365–391. PMID: 17264732.10. Ok SH, Bae SI, Kwon SC, Park JC, Kim WC, Park KE, et al. Bupivacaine-induced vasodilation is mediated by decreased calcium sensitization in isolated endothelium-denuded rat aortas precontracted with phenylephrine. Korean J Pain. 2014; 27:229–238. PMID: 25031808.
Article11. Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 1: basic mechanisms controlling cytosolic Ca2+ concentration and the Ca2+-dependent regulation of vascular tone. J Anesth. 2007; 21:220–231. PMID: 17458652.
Article12. Ok SH, Byon HJ, Kwon SC, Park J, Lee Y, Hwang Y, et al. Lipid emulsion inhibits vasodilation induced by a toxic dose of bupivacaine via attenuated dephosphorylation of myosin phosphatase target subunit 1 in isolated rat aorta. Int J Med Sci. 2015; 12:958–967. PMID: 26664257.
Article13. Sung HJ, Choi MJ, Ok SH, Lee SH, Hwang IJ, Kim HS, et al. Mepivacaine-induced contraction is attenuated by endothelial nitric oxide release in isolated rat aorta. Can J Physiol Pharmacol. 2012; 90:863–872. PMID: 22702717.
Article14. Lee HM, Ok SH, Sung HJ, Eun SY, Kim HJ, Lee SH, et al. Mepivacaine-induced contraction involves phosphorylation of extracellular signal-regulated kinase through activation of the lipoxygenase pathway in isolated rat aortic smooth muscle. Can J Physiol Pharmacol. 2013; 91:285–294. PMID: 23627840.
Article15. Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989; 10:320–325. PMID: 2686129.
Article16. Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000; 522:177–185. PMID: 10639096.
Article17. Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001; 22:32–39. PMID: 11165670.
Article18. Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000; 275:9897–9900. PMID: 10744661.
Article19. Koyama M, Ito M, Feng J, Seko T, Shiraki K, Takase K, et al. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett. 2000; 475:197–200. PMID: 10869555.
Article20. Partownavid P, Umar S, Li J, Rahman S, Eghbali M. Fatty-acid oxidation and calcium homeostasis are involved in the rescue of bupivacaine-induced cardiotoxicity by lipid emulsion in rats. Crit Care Med. 2012; 40:2431–2437. PMID: 22647409.
Article21. Fettiplace MR, Ripper R, Lis K, Lin B, Lang J, Zider B, et al. Rapid cardiotonic effects of lipid emulsion infusion*. Crit Care Med. 2013; 41:e156–e162. PMID: 23531591.22. Fettiplace MR, Lis K, Ripper R, Kowal K, Pichurko A, Vitello D, et al. Multi-modal contributions to detoxification of acute pharmacotoxicity by a triglyceride micro-emulsion. J Control Release. 2015; 198:62–70. PMID: 25483426.
Article23. Lee SH, Sung HJ, Ok SH, Yu J, Choi MJ, Lim JS, et al. Lipid emulsions enhance the norepinephrine-mediated reversal of local anesthetic-induced vasodilation at toxic doses. Yonsei Med J. 2013; 54:1524–1532. PMID: 24142661.
Article24. Ok SH, Yu J, Lee Y, Cho H, Shin IW, Sohn JT. Lipid emulsion attenuates apoptosis induced by a toxic dose of bupivacaine in H9c2 rat cardiomyoblast cells. Hum Exp Toxicol. 2016; 35:929–937. PMID: 26437793.
Article25. Aumeier C, Kasdorf B, Gruber M, Busse H, Wiese CH, Zink W, et al. Lipid emulsion pretreatment has different effects on mepivacaine and bupivacaine cardiac toxicity in an isolated rat heart model. Br J Anaesth. 2014; 112:735–741. PMID: 24169820.
Article26. Zausig YA, Zink W, Keil M, Sinner B, Barwing J, Wiese CH, et al. Lipid emulsion improves recovery from bupivacaine-induced cardiac arrest, but not from ropivacaine- or mepivacaine-induced cardiac arrest. Anesth Analg. 2009; 109:1323–1326. PMID: 19762764.
Article27. McLure HA, Rubin AP. Review of local anaesthetic agents. Minerva Anestesiol. 2005; 71:59–74. PMID: 15714182.28. Park J, Kim YA, Han JY, Jin S, Ok SH, Sohn JT, et al. Lipofundin® MCT/LCT 20% increase left ventricular systolic pressure in an ex vivo rat heart model via increase of intracellular calcium level. Korean J Anesthesiol. 2016; 69:57–62. PMID: 26885303.
Article29. Ward JP, Knock GA, Snetkov VA, Aaronson PI. Protein kinases in vascular smooth muscle tone--role in the pulmonary vasculature and hypoxic pulmonary vasoconstriction. Pharmacol Ther. 2004; 104:207–231. PMID: 15556675.
Article30. Neal JM, Bernards CM, Butterworth JF 4th, Di Gregorio G, Drasner K, Hejtmanek MR, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010; 35:152–161. PMID: 20216033.
Article31. Lee JH, Lee KN, Moon JI. Respiratory arrest during epidural infusion of bupivacaine and morphine. J Korean Pain Soc. 1995; 8:386–389.32. Santos AC, DeArmas PI. Systemic toxicity of levobupivacaine, bupivacaine, and ropivacaine during continuous intravenous infusion to nonpregnant and pregnant ewes. Anesthesiology. 2001; 95:1256–1264. PMID: 11684998.
Article33. Shin IW, Hah YS, Kim C, Park J, Shin H, Park KE, et al. Systemic blockage of nitric oxide synthase by L-NAME increases left ventricular systolic pressure, which is not augmented further by Intralipid®. Int J Biol Sci. 2014; 10:367–376. PMID: 24719554.
Article34. Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001; 38:1–12.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lipid emulsion-mediated reversal of toxic-dose aminoamide local anesthetic-induced vasodilation in isolated rat aorta
- Lipid Emulsions Enhance the Norepinephrine-Mediated Reversal of Local Anesthetic-Induced Vasodilation at Toxic Doses
- Bupivacaine-induced Vasodilation Is Mediated by Decreased Calcium Sensitization in Isolated Endothelium-denuded Rat Aortas Precontracted with Phenylephrine
- Effects of Bupivacaine on the Membrane Potential and Intracellular Na
- Effect of Clonidine Pretreatment on the Cardiovascular Toxicity Induced by Inrravenous Bupivacaine in Rabbits