Yonsei Med J.
2013 Nov;54(6):1524-1532. 10.3349/ymj.2013.54.6.1524.
Lipid Emulsions Enhance the Norepinephrine-Mediated Reversal of Local Anesthetic-Induced Vasodilation at Toxic Doses
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, Jinju, Korea.
- 2Department of Anesthesiology and Pain Medicine, Institute of Health Sciences, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea. jtsohn@nongae.gsnu.ac.kr
- 3Department of Oral and Maxillofacial Surgery, Gyeongsang National University Hospital, Jinju, Korea.
- 4Department of Biological Sciences, Pusan National University, Busan, Korea.
- KMID: 1798153
- DOI: http://doi.org/10.3349/ymj.2013.54.6.1524
Abstract
- PURPOSE
Intravenous lipid emulsions have been used to treat the systemic toxicity of local anesthetics. The goal of this in vitro study was to examine the effects of lipid emulsions on the norepinephrine-mediated reversal of vasodilation induced by high doses of levobupivacaine, ropivacaine, and mepivacaine in isolated endothelium-denuded rat aorta, and to determine whether such effects are associated with the lipid solubility of local anesthetics.
MATERIALS AND METHODS
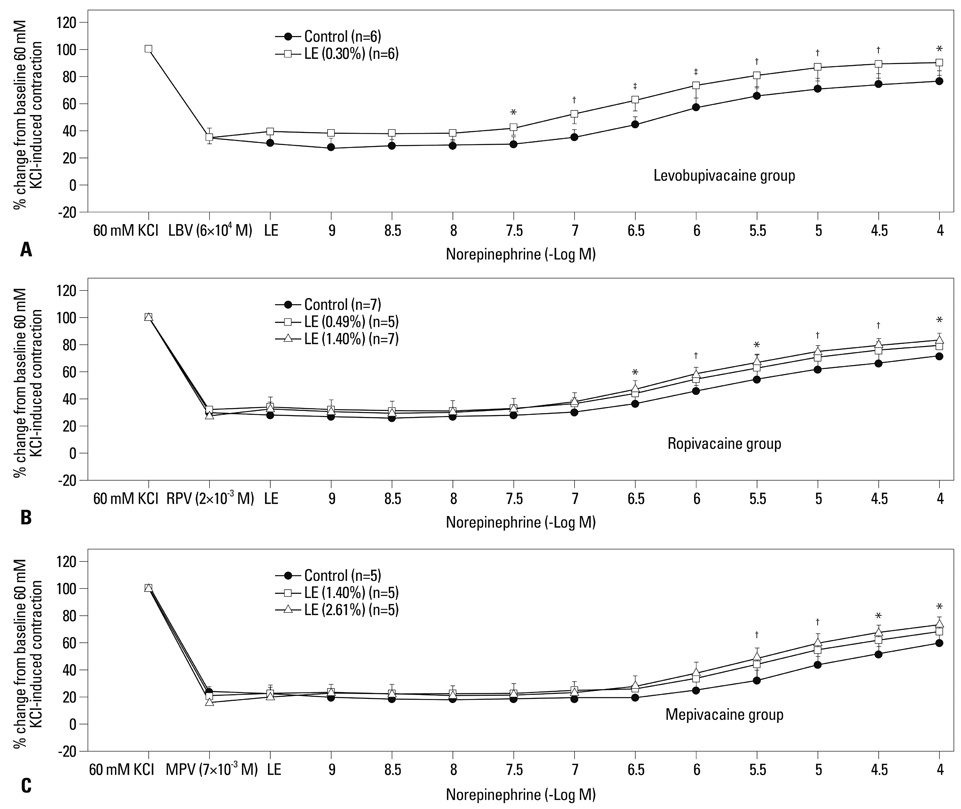
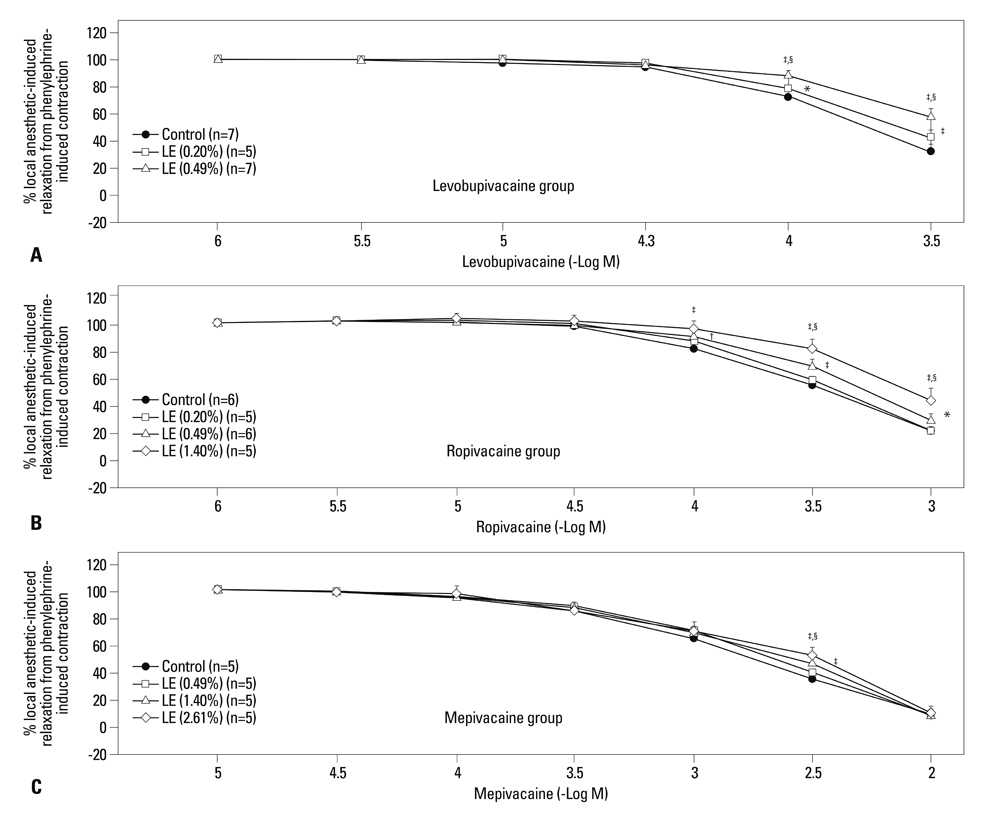
The effects of lipid emulsions (0.30, 0.49, 1.40, and 2.61%) on norepinephrine concentration-responses in high-dose local anesthetic (6x10-4 M levobupivacaine, 2x10-3 M ropivacaine, and 7x10-3 M mepivacaine)-induced vasodilation of isolated aorta precontracted with 60 mM KCl were assessed. The effects of lipid emulsions on local anesthetic- and diltiazem-induced vasodilation in isolated aorta precontracted with phenylephrine were also assessed.
RESULTS
Lipid emulsions (0.30%) enhanced norepinephrine-induced contraction in levobupivacaine-induced vasodilation, whereas 1.40 and 2.61% lipid emulsions enhanced norepinephrine-induced contraction in both ropivacaine- and mepivacaine-induced vasodilation, respectively. Lipid emulsions (0.20, 0.49 and 1.40%) inhibited vasodilation induced by levobupivacaine and ropivacaine, whereas 1.40 and 2.61% lipid emulsions slightly attenuated mepivacaine (3x10-3 M)-induced vasodilation. In addition, lipid emulsions attenuated diltiazem-induced vasodilation. Lipid emulsions enhanced norepinephrine-induced contraction in endothelium-denuded aorta without pretreatment with local anesthetics.
CONCLUSION
Taken together, these results suggest that lipid emulsions enhance the norepinephrine-mediated reversal of local anesthetic-induced vasodilation at toxic anesthetic doses and inhibit local anesthetic-induced vasodilation in a manner correlated with the lipid solubility of a particular local anesthetic.
Keyword
MeSH Terms
-
Amides/adverse effects
Anesthetics, Local/*adverse effects
Animals
Bupivacaine/adverse effects/analogs & derivatives
Emulsions/*chemistry/*therapeutic use
Lipids/*chemistry
Male
Mepivacaine/adverse effects
Norepinephrine/*therapeutic use
Rats
Rats, Sprague-Dawley
Vasodilation/*drug effects
Amides
Anesthetics, Local
Bupivacaine
Emulsions
Lipids
Mepivacaine
Norepinephrine
Figure
Cited by 1 articles
-
Lipid emulsion inhibits vasodilation induced by a toxic dose of bupivacaine by suppressing bupivacaine-induced PKC and CPI-17 dephosphorylation but has no effect on vasodilation induced by a toxic dose of mepivacaine
Hyunhoo Cho, Seong Ho Ok, Seong Chun Kwon, Soo Hee Lee, Jiseok Baik, Sebin Kang, Jiah Oh, Ju-Tae Sohn
Korean J Pain. 2016;29(4):229-238. doi: 10.3344/kjp.2016.29.4.229.
Reference
-
1. Charbonneau H, Marcou TA, Mazoit JX, Zetlaoui PJ, Benhamou D. Early use of lipid emulsion to treat incipient mepivacaine intoxication. Reg Anesth Pain Med. 2009; 34:277–278.
Article2. Schaeffer E, Rayaud L, Landy C, Boulland P, Favier JC. [Local anaesthetics intoxication, during ultrasound-guided axillary plexus block, treated by Intralipide]. Ann Fr Anesth Reanim. 2010; 29:929–930.3. Sakai T, Manabe W, Kamitani T, Takeyama E, Nakano S. [Ropivacaine-induced late-onset systemic toxicity after transversus abdominis plane block under general anesthesia: successful reversal with 20% lipid emulsion]. Masui. 2010; 59:1502–1505.4. Dix SK, Rosner GF, Nayar M, Harris JJ, Guglin ME, Winterfield JR, et al. Intractable cardiac arrest due to lidocaine toxicity successfully resuscitated with lipid emulsion. Crit Care Med. 2011; 39:872–874.
Article5. Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med. 2003; 28:198–202.
Article6. Foxall G, McCahon R, Lamb J, Hardman JG, Bedforth NM. Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia. 2007; 62:516–518.
Article7. Ok SH, Sohn JT, Baik JS, Kim JG, Park SS, Sung HJ, et al. Lipid emulsion reverses Levobupivacaine-induced responses in isolated rat aortic vessels. Anesthesiology. 2011; 114:293–301.
Article8. Baik JS, Sohn JT, Ok SH, Kim JG, Sung HJ, Park SS, et al. Levobupivacaine-induced contraction of isolated rat aorta is calcium dependent. Can J Physiol Pharmacol. 2011; 89:467–476.
Article9. Sung HJ, Choi MJ, Ok SH, Lee SH, Hwang IJ, Kim HS, et al. Mepivacaine-induced contraction is attenuated by endothelial nitric oxide release in isolated rat aorta. Can J Physiol Pharmacol. 2012; 90:863–872.
Article10. Li B, Yan J, Shen Y, Li B, Hu Z, Ma Z. Association of sustained cardiovascular recovery with epinephrine in the delayed lipid-based resuscitation from cardiac arrest induced by bupivacaine overdose in rats. Br J Anaesth. 2012; 108:857–863.
Article11. Harvey M, Cave G, Prince G, Lahner D. Epinephrine injection in lipid-based resuscitation from bupivacaine-induced cardiac arrest: transient circulatory return in rabbits. Anesth Analg. 2010; 111:791–796.
Article12. Liu L, Xia Y, Chen Y, Wang Q, Shi T, Wang F, et al. The comparative effects of lipid, epinephrine, and their combination in the reversal of bupivacaine-induced asystole in the isolated rat heart. Anesth Analg. 2012; 114:886–893.
Article13. Hiller DB, Gregorio GD, Ripper R, Kelly K, Massad M, Edelman L, et al. Epinephrine impairs lipid resuscitation from bupivacaine overdose: a threshold effect. Anesthesiology. 2009; 111:498–505.14. Hollenberg SM. Vasoactive drugs in circulatory shock. Am J Respir Crit Care Med. 2011; 183:847–855.
Article15. Heavner JE, Pitkänen MT, Shi B, Rosenberg PH. Resuscitation from bupivacaine-induced asystole in rats: comparison of different cardioactive drugs. Anesth Analg. 1995; 80:1134–1139.16. Ok SH, Bae SI, Shim HS, Sohn JT. Dexmedetomidine-induced contraction of isolated rat aorta is dependent on extracellular calcium concentration. Korean J Anesthesiol. 2012; 63:253–259.
Article17. Sung HJ, Ok SH, Sohn JY, Son YH, Kim JK, Lee SH, et al. Vasoconstriction potency induced by aminoamide local anesthetics correlates with lipid solubility. J Biomed Biotechnol. 2012; 2012:170958.
Article18. Cassidy SL, Lympany PA, Henry JA. Lipid solubility of a series of drugs and its relevance to fatal poisoning. J Pharm Pharmacol. 1988; 40:130–132.
Article19. Cox B, Durieux ME, Marcus MA. Toxicity of local anaesthetics. Best Pract Res Clin Anaesthesiol. 2003; 17:111–136.
Article20. Zapata-Sudo G, Trachez MM, Sudo RT, Nelson TE. Is comparative cardiotoxicity of S(-) and R(+) bupivacaine related to enantiomer-selective inhibition of L-type Ca(2+) channels? Anesth Analg. 2001; 92:496–501.
Article21. Sung HJ, Sohn JT, Park JY, Hwang EM, Baik JS, Ogawa K. Direct effect of ropivacaine involves lipoxygenase pathway activation in rat aortic smooth muscle. Can J Anaesth. 2009; 56:298–306.
Article22. Strichartz GR, Sanchez V, Arthur GR, Chafetz R, Martin D. Fundamental properties of local anesthetics. II. Measured octanol:buffer partition coefficients and pKa values of clinically used drugs. Anesth Analg. 1990; 71:158–170.23. Ozcan MS, Weinberg G. Update on the use of lipid emulsions in local anesthetic systemic toxicity: a focus on differential efficacy and lipid emulsion as part of advanced cardiac life support. Int Anesthesiol Clin. 2011; 49:91–103.
Article24. Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci U S A. 1992; 89:6452–6456.
Article25. Weinberg GL. Lipid emulsion infusion: resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012; 117:180–187.26. Waring WS. Intravenous lipid administration for drug-induced toxicity: a critical review of the existing data. Expert Rev Clin Pharmacol. 2012; 5:437–444.
Article27. Montiel V, Gougnard T, Hantson P. Diltiazem poisoning treated with hyperinsulinemic euglycemia therapy and intravenous lipid emulsion. Eur J Emerg Med. 2011; 18:121–123.
Article28. Groban L. Central nervous system and cardiac effects from long-acting amide local anesthetic toxicity in the intact animal model. Reg Anesth Pain Med. 2003; 28:3–11.
Article29. Santos AC, Arthur GR, Pedersen H, Morishima HO, Finster M, Covino BG. Systemic toxicity of ropivacaine during ovine pregnancy. Anesthesiology. 1991; 75:137–141.
Article30. Tanoubi I, Vialles N, Cuvillon P, Ripart J. [Systemic toxicity with mepivacaine following axillary block in a patient with terminal kidney failure]. Ann Fr Anesth Reanim. 2006; 25:33–35.31. Mertes N, Grimm H, Fürst P, Stehle P. Safety and efficacy of a new parenteral lipid emulsion (SMOFlipid) in surgical patients: a randomized, double-blind, multicenter study. Ann Nutr Metab. 2006; 50:253–259.
Article32. Weinberg GL, Ripper R, Murphy P, Edelman LB, Hoffman W, Strichartz G, et al. Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesth Pain Med. 2006; 31:296–303.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lipid emulsion-mediated reversal of toxic-dose aminoamide local anesthetic-induced vasodilation in isolated rat aorta
- Lipid Emulsion in the Successful Resuscitation of Local Anesthetic Toxicity after Ankle Block
- Lipid emulsion treatment of systemic toxicity induced by local anesthetics or other drugs
- Lipid emulsion inhibits vasodilation induced by a toxic dose of bupivacaine by suppressing bupivacaine-induced PKC and CPI-17 dephosphorylation but has no effect on vasodilation induced by a toxic dose of mepivacaine
- Influence of Phentolamine on the centrally induced Renal effects of Norepinephrine and Dopamine in the Rabbit