Korean J Pain.
2014 Jul;27(3):229-238. 10.3344/kjp.2014.27.3.229.
Bupivacaine-induced Vasodilation Is Mediated by Decreased Calcium Sensitization in Isolated Endothelium-denuded Rat Aortas Precontracted with Phenylephrine
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Institute of Health Sciences, Gyeongsang National University School of Medicine, Gyeongsang National University Hospital, Jinju, Korea. jtsohn@nongae.gsnu.ac.kr
- 2Department of Anesthesiology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 3Department of Physiology, Kwandong University College of Medicine, Gangneung, Korea.
- 4Department of Anesthesiology and Pain Medicine, Gyeongsang National University Hospital, Jinju, Korea.
- 5Department of Oral and Maxillofacial Surgery, Gyeongsang National University Hospital, Jinju, Korea.
- KMID: 2278228
- DOI: http://doi.org/10.3344/kjp.2014.27.3.229
Abstract
- BACKGROUND
A toxic dose of bupivacaine produces vasodilation in isolated aortas. The goal of this in vitro study was to investigate the cellular mechanism associated with bupivacaine-induced vasodilation in isolated endotheliumdenuded rat aortas precontracted with phenylephrine.
METHODS
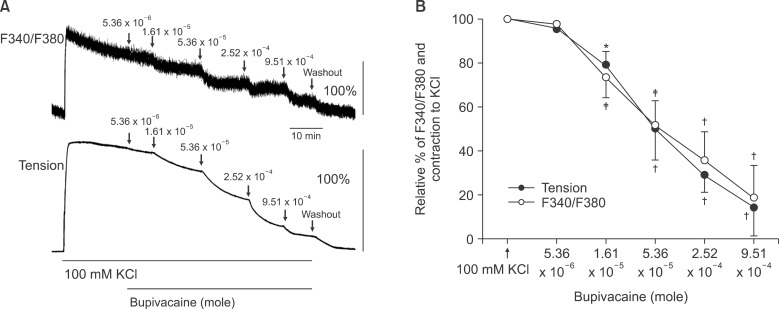
Isolated endothelium-denuded rat aortas were suspended for isometric tension recordings. The effects of nifedipine, verapamil, iberiotoxin, 4-aminopyridine, barium chloride, and glibenclamide on bupivacaine concentration-response curves were assessed in endothelium-denuded aortas precontracted with phenylephrine. The effect of phenylephrine and KCl used for precontraction on bupivacaine-induced concentration-response curves was assessed. The effects of verapamil on phenylephrine concentration-response curves were assessed. The effects of bupivacaine on the intracellular calcium concentration ([Ca2+]i) and tension in aortas precontracted with phenylephrine were measured simultaneously with the acetoxymethyl ester of a fura-2-loaded aortic strip.
RESULTS
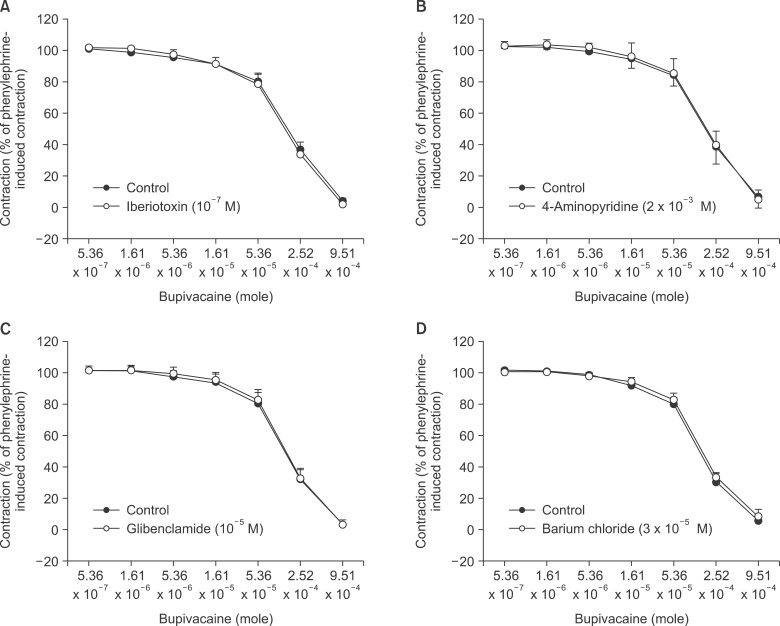
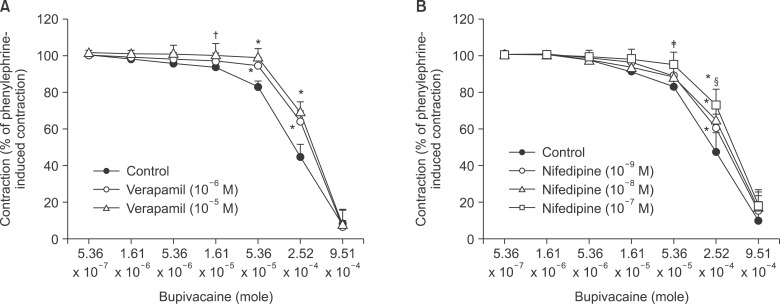
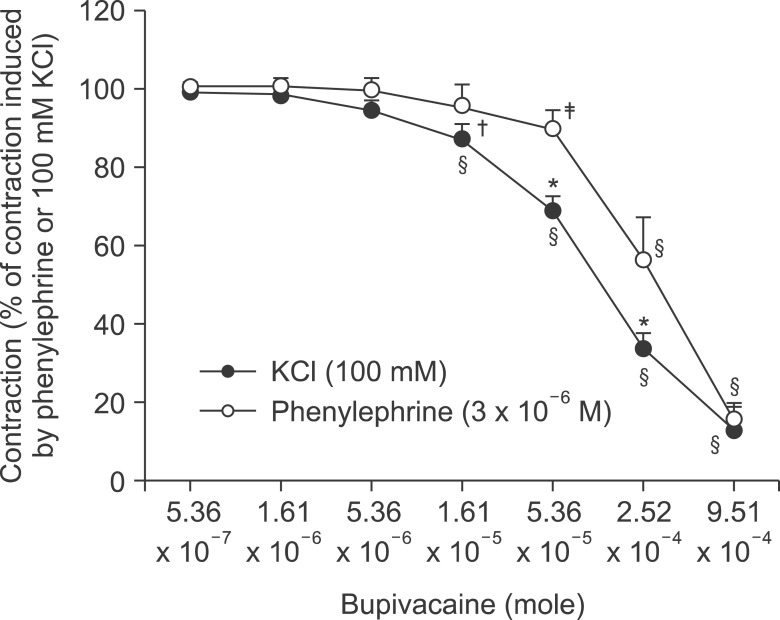
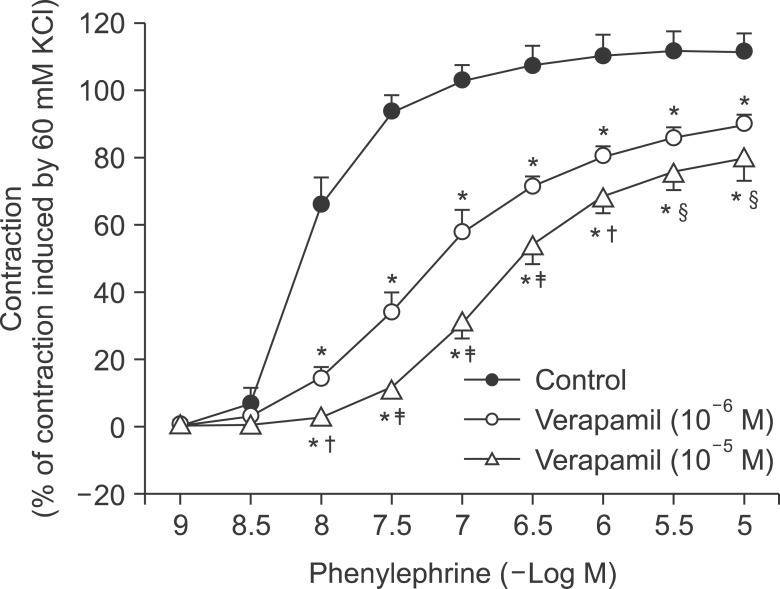
Pretreatment with potassium channel inhibitors had no effect on bupivacaine-induced relaxation in the endothelium-denuded aortas precontracted with phenylephrine, whereas verapamil or nifedipine attenuated bupivacaine-induced relaxation. The magnitude of the bupivacaine-induced relaxation was enhanced in the 100 mM KCl-induced precontracted aortas compared with the phenylephrine-induced precontracted aortas. Verapamil attenuated the phenylephrine-induced contraction. The magnitude of the bupivacaine-induced relaxation was higher than that of the bupivacaine-induced [Ca2+]i decrease in the aortas precontracted with phenylephrine.
CONCLUSIONS
Taken together, these results suggest that toxic-dose bupivacaine-induced vasodilation appears to be mediated by decreased calcium sensitization in endothelium-denuded aortas precontracted with phenylephrine. In addition, potassium channel inhibitors had no effect on bupivacaine-induced relaxation. Toxic-dose bupivacaine- induced vasodilation may be partially associated with the inhibitory effect of voltage-operated calcium channels.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Lipid emulsion inhibits vasodilation induced by a toxic dose of bupivacaine by suppressing bupivacaine-induced PKC and CPI-17 dephosphorylation but has no effect on vasodilation induced by a toxic dose of mepivacaine
Hyunhoo Cho, Seong Ho Ok, Seong Chun Kwon, Soo Hee Lee, Jiseok Baik, Sebin Kang, Jiah Oh, Ju-Tae Sohn
Korean J Pain. 2016;29(4):229-238. doi: 10.3344/kjp.2016.29.4.229.
Reference
-
1. Beilin Y, Halpern S. Focused review: ropivacaine versus bupivacaine for epidural labor analgesia. Anesth Analg. 2010; 111:482–487. PMID: 20529986.2. Szocik JF, Gardner CA, Webb RC. Inhibitory effects of bupivacaine and lidocaine on adrenergic neuroeffector junctions in rat tail artery. Anesthesiology. 1993; 78:911–917. PMID: 8489063.
Article3. Hahnenkamp K, Nollet J, Strümper D, Halene T, Rathman P, Mortier E, et al. Bupivacaine inhibits thromboxane A2-induced vasoconstriction in rat thoracic aorta. Anesth Analg. 2004; 99:97–102. PMID: 15281511.
Article4. Ok SH, Park CS, Kim HJ, Lee SH, Choi BH, Eun SY, et al. Effect of two lipid emulsions on reversing high-dose levobupivacaine-induced reduced vasoconstriction in the rat aortas. Cardiovasc Toxicol. 2013; 13:370–380. PMID: 23877627.
Article5. Sung HJ, Ok SH, Sohn JY, Son YH, Kim JK, Lee SH, et al. Vasoconstriction potency induced by aminoamide local anesthetics correlates with lipid solubility. J Biomed Biotechnol. 2012; 2012:170958. PMID: 22778542.
Article6. Shim HS, Ok SH, Lee SH, Kwon SC, Sohn JT. Protein kinases participate in the contraction in response to levobupivacaine in the rat aorta. Eur J Pharmacol. 2012; 677:131–137. PMID: 22222819.
Article7. Baik JS, Sohn JT, Ok SH, Kim JG, Sung HJ, Park SS, et al. Levobupivacaine-induced contraction of isolated rat aorta is calcium dependent. Can J Physiol Pharmacol. 2011; 89:467–476. PMID: 21812525.
Article8. Ok SH, Sohn JT, Baik JS, Kim JG, Park SS, Sung HJ, et al. Lipid emulsion reverses Levobupivacaine-induced responses in isolated rat aortic vessels. Anesthesiology. 2011; 114:293–301. PMID: 21239969.
Article9. Choi YS, Jeong YS, Ok SH, Shin IW, Lee SH, Park JY, et al. The direct effect of levobupivacaine in isolated rat aorta involves lipoxygenase pathway activation and endothelial nitric oxide release. Anesth Analg. 2010; 110:341–349. PMID: 19955508.
Article10. Ok SH, Han JY, Lee SH, Shin IW, Lee HK, Chung YK, et al. Lipid emulsion-mediated reversal of toxic-dose aminoamide local anesthetic-induced vasodilation in isolated rat aorta. Korean J Anesthesiol. 2013; 64:353–359. PMID: 23646246.
Article11. Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008; 44:65–81. PMID: 18552454.12. Shin IW, Sohn JT, Kim HJ, Kim C, Lee HK, Chang KC, et al. Etomidate attenuates phenylephrine-induced contraction in isolated rat aorta. Can J Anaesth. 2005; 52:927–934. PMID: 16251557.
Article13. Sohn JT, Park KE, Kim C, Jeong YS, Shin IW, Lee HK, et al. Alfentanil attenuates phenylephrine-induced contraction in rat aorta. Eur J Anaesthesiol. 2007; 24:276–282. PMID: 17054815.
Article14. Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 2: regulatory mechanisms modulating Ca2+ mobilization and/or myofilament Ca2+ sensitivity in vascular smooth muscle cells. J Anesth. 2007; 21:232–242. PMID: 17458653.
Article15. Ok SH, Kwon SC, Yeol Han J, Yu J, Shin IW, Lee HK, et al. Mepivacaine-induced contraction involves increased calcium sensitization mediated via Rho kinase and protein kinase C in endothelium-denuded rat aorta. Eur J Pharmacol. 2014; 723:185–193. PMID: 24333215.
Article16. Kaya T, Gursoy S, Karadas B, Sarac B, Fafali H, Soydan AS. High-concentration tramadol-induced vasodilation in rabbit aorta is mediated by both endothelium-dependent and -independent mechanisms. Acta Pharmacol Sin. 2003; 24:385–389. PMID: 12740170.17. Subramaniam G, Achike FI, Mustafa MR. Effect of acidosis on the mechanism(s) of insulin-induced vasorelaxation in normal Wistar-Kyoto (WKY) rat aorta. Regul Pept. 2009; 155:70–75. PMID: 19362578.
Article18. Xue YL, Shi HX, Murad F, Bian K. Vasodilatory effects of cinnamaldehyde and its mechanism of action in the rat aorta. Vasc Health Risk Manag. 2011; 7:273–280. PMID: 21603596.19. Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989; 10:320–325. PMID: 2686129.
Article20. Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005; 83:215–242. PMID: 15870837.
Article21. Kitazawa T, Kitazawa K. Size-dependent heterogeneity of contractile Ca2+ sensitization in rat arterial smooth muscle. J Physiol. 2012; 590:5401–5423. PMID: 22930267.
Article22. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, et al. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997; 49:157–230. PMID: 9228665.23. Huang Y, Ho IH. Separate activation of intracellular Ca2+ release, voltage-dependent and receptor-operated Ca2+ channels in the rat aorta. Chin J Physiol. 1996; 39:1–8. PMID: 8902298.24. Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001; 38:1–12. PMID: 11173989.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Propofol on Phenylephrine Induced Vascular Smooth Muscle Contraction in Rat Thoracic Aorta
- Lipid emulsion-mediated reversal of toxic-dose aminoamide local anesthetic-induced vasodilation in isolated rat aorta
- Lipid Emulsions Enhance the Norepinephrine-Mediated Reversal of Local Anesthetic-Induced Vasodilation at Toxic Doses
- Mechanism of UV light-induced photorelaxation in isolated rat aorta
- Effects of L-Arginine and N-Nitro-L-Arginine Metheylester Treatment on Vasodilation of Propofol in Rat Thoracic Aortic Rings