J Korean Acad Prosthodont.
2008 Dec;46(6):620-627.
Cellular responses on anodized titanium discs coated with 1 alpha,25-dihydroxyvitamin D3 incorporated Poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles
- Affiliations
-
- 1Department of Prosthodontics and Dental Research Institute, School of Dentistry, Seoul National University, Korea. ksy0617@snu.ac.kr
- 2Department of Prosthodontics, Asan Medical Center, College of Medicine, University of Ulsan, Korea.
Abstract
-
STATEMENT OF PROBLEM: A biochemical approach for surface modification has offered an alternative for physicochemical and morphological methods to obtain desirable bone-implant interfaces.
PURPOSE: The purpose of the present study was to investigate cell responses to poly (D,L-lactide-co-glycolide) (PLGA)/1alpha, 25-(OH)2D3 coating with reference to cellular proliferation and differentiation in vitro.
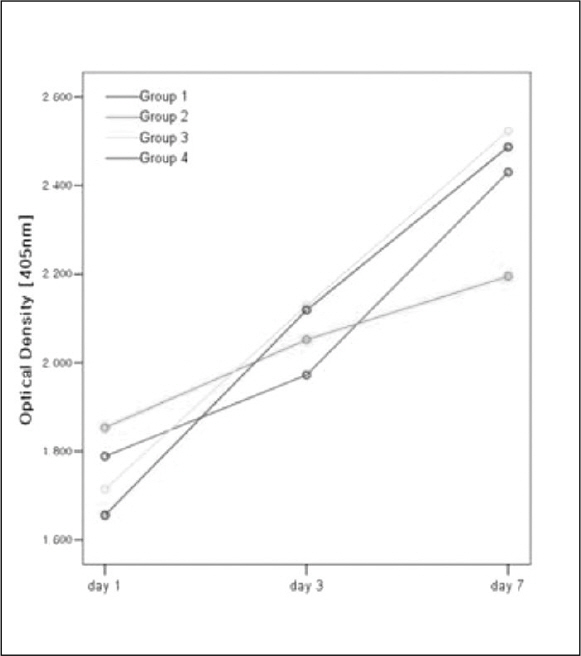
MATERIAL AND METHODS: 96 titanium discs were fabricated and divided into four groups. Group 1 was anodized under 300 V as control. Group 2, 3 and 4 were anodized then coated with 3 ml PLGA/1alpha, 25-(OH)2D3 solutions. Amount of the solutions were 2 ul, 20 ul and 200ul respectively. The osteoblast-like Human Osteogenic Sarcoma (HOS) cells were seeded and cultured for 1, 3 and 7 days. MTSbased cell proliferation assay and ALPase activity test were carried out.
RESULTS
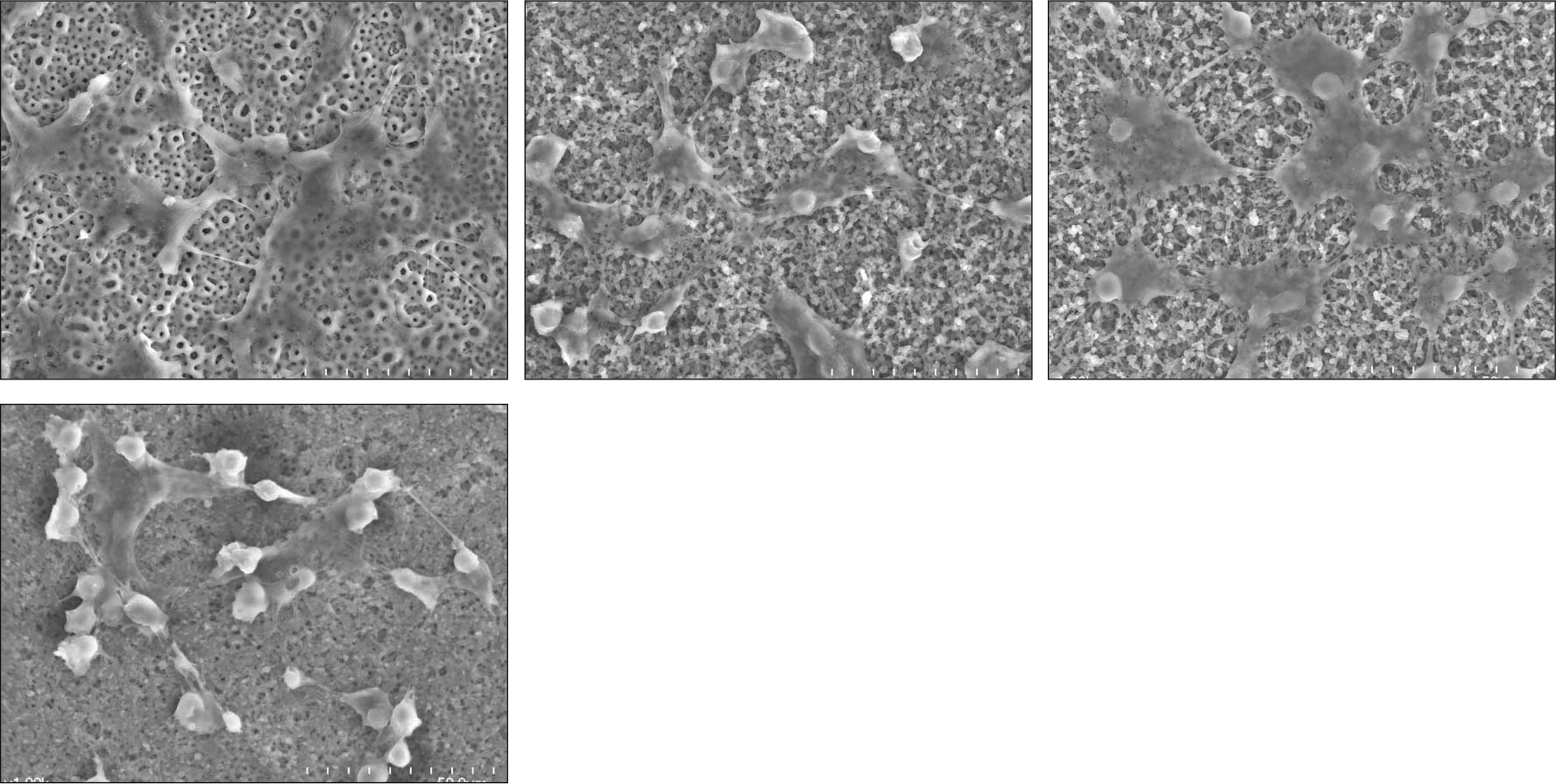
PLGA nanoparticles were observed as fine, smooth and round and HOS cells attached to the anodized surfaces through strand-like and sheet-like filopodia. After 3 days of culture, the dendritic filopodia were exaggerated and sheet-like cytoplasmic projections covered the coated titanium surfaces. After 3 days of culture, all of the groups showed increased cellular proliferation and the lowest proliferation rate was measured on group 2. Higher amount of incorporated 1 alpha, 25-(OH)2D3 (Group 3 and 4) improved cellular proliferation but the differences were not significant statistically (P > .05). But they increased the rate of ALP activities than the control group at day 3 (P < .05).
CONCLUSION
Biodegradable PLGA nanoparticles incorporated with vitamin D metabolite positively affected proliferation and differentiation of cells on the anodized titanium surface.
Keyword
MeSH Terms
Figure
Reference
-
1.Ito Y., Kajihara M., Imanishi Y. Materials for enhancing cell adhesion by immobilization of cell-adhesive peptide. J Biomed Mater Res. 1991. 25:1325–37.
Article2.van Driel M., Koedam M., Buurman CJ., Roelse M., Weyts F., Chiba H., Uitter linden AG., Pols HA., van Leeuwen JP. Evidence that both 1α,25-Dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem. 2006. 99:922–35.
Article3.Miyahara T., Simoura T., Osahune N., Uchida Y., Sakuma T., Nemoto N., Kozakai A., Takamura T., Yamazaki R., Higuchi S., Chiba H., Iba K., Sawada N. A highly potent 26,27-Hexafluoro-1α,25-dihydroxyvitamin D3 on calcification in SV40-transformed human fetal osteoblastic cells. Calcif Tissue Int. 2002. 70:488–95.4.Puleo DA., Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999. 20:2311–21.
Article5.Agrawal CM., Niederauer GG., Athanasiou KA. Fabrication and characterization of PLA-PGA orthopaedic implants. Tissue Eng. 1995. 1:241–52.6.Kulkarni RK., Moore G., Hegyeli AF., Leonard F. Biodegradable poly(lactic acid) polymers. J Biomed Mater Res. 1971. 5:169–81.
Article7.Panyam J., Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003. 55:329–47.
Article8.Li LH., Kong YM., Kim HW., Kim YW., Kim HE., Heo SJ., Koak JY. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004. 25:2867–75.
Article9.Choi JW., Heo SJ., Koak JY., Kim SK., Lim YJ., Kim SH., Lee JB. Biological responses of anodized titanium implants under different current voltages. J Oral Rehabil. 2006. 33:889–97.
Article10.Woosley J., Turnbull R., Kim K. Field injection electrostatic spraying of liquid hydrogen. J Appl Phys. 1988. 64:4278–85.
Article11.Kim K., Turnbull RJ. Generation of charged drops of insulating liquids by electrostatic spraying. J Appl Phys. 1976. 47:1964–9.
Article12.Cory AH., Owen TC., Barltrop JA., Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991. 3:207–12.
Article13.van Leeuwen JP., van Driel M., van den Bemd GJ., Pols HA. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit Rev Eukaryot Gene Expr. 2001. 11:199–226.14.Siggelkow H., Schulz H., Kaesler S., Benzler K., Atkinson MJ., Hufner M. 1,25 dihydroxyvitamin-D3 attenuates the confluence-dependent differences in the osteoblast characteristic proteins alkaline phosphatase, procollagen I peptide, and osteocalcin. Calcif Tissue Int. 1999. 64:414–21.
Article15.Raz P., Lohmann CH., Turner J., Wang L., Poythress N., Blanchard C., Boyan BD., Schwartz Z. 1α,25-(OH)2D3 Regulation of integrin expression is substrate dependent. J Biomed Mater Res A. 2004. 71:217–25.
Article16.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) devices. Biomaterials. 2000. 21:2475–90.17.Shive MS., Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997. 28:5–24.18.Owen TA., Aronow M., Shalhoub V., Barone LM., Wilming L., TAssinari MS., Kennedy MB., Pockwines S., Lian JB., Stein GS. Progressive development of the rat osteoblast phenotype in vitro: Reciprocal relationship in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990. 143:420–30.19.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000. 21:667–81.
Article20.Li LH., Kim HW., Lee SH., Kong YM., Kim HE. Biocompatibility of titanium implants modified by mi-croarc oxidation and hydroxyapatite coating. J Biomed Mater Res A. 2005. 73:48–54.
Article21.Boyan BD., Batzer R., Kieswetter K., Liu Y., Cochran DL., Szmuckler-Moncler S., Dean DD., Schwartz Z. Titanium surface roughness alters responsiveness of MG63 os-teoblastlike cells to 1α,25-(OH)2D3. J Biomed Mater Res. 1998. 39:77–85.
Article22.Zhao G., Schwartz Z., Wieland M., Rupp F., Geis-Gerstorfer J., Cochran DL., Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005. 74:49–58.
Article23.Lohmann CH., Bonewald LF., Sisk MA., Sylvia VL., Cochran DL., Dean DD., Boyan BD., Schwartz Z. Maturation State Determines the Response of Osteogenic Cells to Surface Roughness and 1,25-Dihydroxyvitamin D3 J Bone Miner Res. 2000. 15:1169–80.24.Lohmann CH., Sagun R Jr. Sylvia VL, Cochran DL, Dean DD, Boyan BD, Schwartz Z. Surface roughness modulates the response of MG63 osteoblast-like cells to 1,25-(OH) 2D3 through regulation of phospholipase A2 activity and activation of proteinkinase A. J Biomed Mater Res. 1999. 47:139–51.25.Franceschi RT., James WM., Zerlauth G. 1α,25-dihydroxy vitamin D3 specific regulation of growth, morphology, and fibronectin in a human osteosarcoma cell line. J Cell Physiol. 1985. 123:401–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cellular Response of Anodized Titanium Surface by Poly(Lactide-co-Glycolide)/Bone Morphogenic Protein-2
- An in vitro study of mesenchymal stem cell proliferation on titanium discs coated with rhTGF-β2/PLGA by electrospray
- A study of mesenchymal stem cell proliferation and surface characteristics of the titanium discs coated with MS275/PLGA by an electrospray
- The sustaining effect of three polymers on the release of chlorhexidine from a controlled release drug device for root canal disinfection
- Wettability and cellular response of UV light irradiated anodized titanium surface