J Adv Prosthodont.
2011 Jun;3(2):63-68. 10.4047/jap.2011.3.2.63.
Wettability and cellular response of UV light irradiated anodized titanium surface
- Affiliations
-
- 1Department of Prosthodontics and Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea. 0504heo@hanmail.net
- KMID: 1975078
- DOI: http://doi.org/10.4047/jap.2011.3.2.63
Abstract
- PURPOSE
The object of this study was to investigate the effect of UV irradiation (by a general commercial UV sterilizer) on anodized titanium surface. Surface characteristics and cellular responses were compared between anodized titanium discs and UV irradiated anodized titanium discs. MATERIALS AND METHODS: Titanium discs were anodized and divided into the following groups: Group 1, anodized (control), and Group 2, anodized and UV irradiated for 24 hours. The surface characteristics including contact angle, roughness, phase of oxide layer, and chemical elemental composition were inspected. The osteoblast-like human osteogenic sarcoma (HOS) cells were cultured on control and test group discs. Initial cellular attachment, MTS-based cell proliferation assay, and ALP synthesis level were compared between the two groups for the evaluation of cellular response.
RESULTS
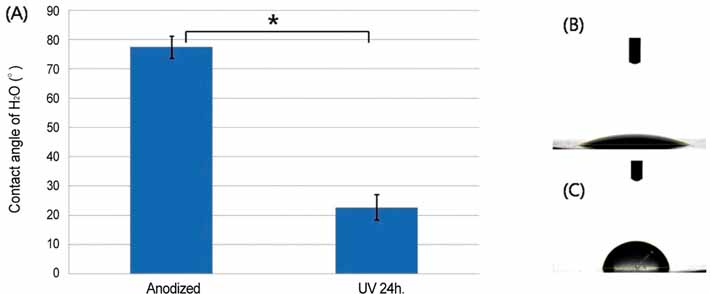
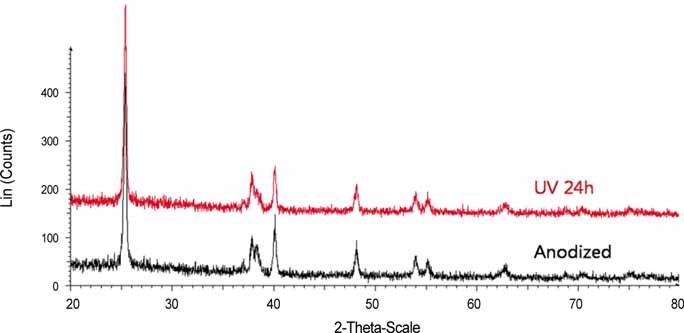
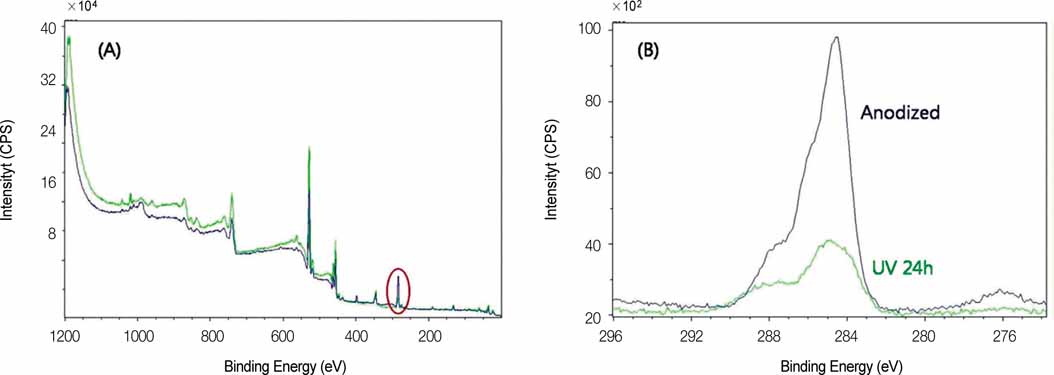
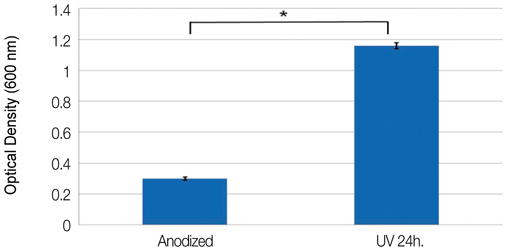
After UV irradiation, the contact angle decreased significantly (P<.001). The surface roughness and phase of oxide layer did not show definite changes, but carbon showed a considerable decrease after UV irradiation. Initial cell attachment was increased in test group (P=.004). Cells cultured on test group samples proliferated more actively (P=.009 at day 2, 5, and 7) and the ALP synthesis also increased in cells cultured on the test group (P=.016 at day 3, P=.009 at day 7 and 14). CONCLUSION: UV irradiation induced enhanced wettability, and increased initial cellular responses of HOS cells on anodized titanium surface.
MeSH Terms
Figure
Reference
-
1. Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977. 16:1–132.2. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004. 17:536–543.3. Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005. 74:49–58.4. Kieswetter K, Schwartz Z, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996. 7:329–345.5. MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials. 2002. 23:1269–1279.6. Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T. Light-induced amphiphilic surfaces. Nature. 1997. 388:431–432.7. Shultz AN, Jang W, Hetherington WM III, Baer DR, Wang LQ, Engelhard MH. Comparative second harmonic generation and X-ray photoelectron spectroscopy studies of the UV creation and O2 healing of Ti3+ defects on (110) rutile TiO2 surfaces. Surf Sci. 1995. 339:114–124.8. Hugenschmidt MB, Gamble L, Campbell CT. The interaction of H2O with a TiO2(110) surface. Surf Sci. 1994. 302:329–340.9. Henderson MA. An HREELS and TPD study of water on TiO2 (110) : the extent of molecular versus dissociative adsorption. Surf Sci. 1996. 355:151–166.10. Bullock EL, Patthey L, Steinemann SG. Clean and hydroxylated rutile TiO2(110) surfaces studied by X-ray photoelectron spectroscopy. Surf Sci. 1996. 352-4:504–510.11. Guillemot F, Porté MC, Labrugére C, Baquey CH. Ti4+ to Ti3+ conversion of TiO2 uppermost layer by low-temperature vacuum annealing: interest for titanium biomedical applications. J Colloid Interface Sci. 2002. 255:75–78.12. Kasemo B, Lausmaa J. Aspects of surface physics on titanium implants. Swed Dent J Suppl. 1985. 28:19–36.13. Zitter H, Plenk H Jr. The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J Biomed Mater Res. 1987. 21:881–896.14. Solar RJ, Pollack SR, Korostoff E. In vitro corrosion testing of titanium surgical implant alloys: an approach to understanding titanium release from implants. J Biomed Mater Res. 1979. 13:217–250.15. Tengvall P, Lundström I. Physico-chemical considerations of titanium as a biomaterial. Clin Mater. 1992. 9:115–134.16. Park KH, Heo SJ, Koak JY, Kim SK, Lee JB, Kim SH, Lim YJ. Osseointegration of anodized titanium implants under different current voltages: a rabbit study. J Oral Rehabil. 2007. 34:517–527.17. Lee JE, Heo SJ, Koak JY, Kim SK, Han CH, Lee SJ. Healing response of cortical and cancellous bone around titanium implants. Int J Oral Maxillofac Implants. 2009. 24:655–662.18. Friberg B, Jemt T. Clinical experience of TiUnite implants: a 5-year cross-sectional, retrospective follow-up study. Clin Implant Dent Relat Res. 2010. 12:e95–e103.19. Li LH, Kong YM, Kim HW, Kim YW, Kim HE, Heo SJ, Koak JY. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004. 25:2867–2875.20. Kilpadi DV, Lemons JE. Surface energy characterization of unalloyed titanium implants. J Biomed Mater Res. 1994. 28:1419–1425.21. Eriksson C, Nygren H, Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials. 2004. 25:4759–4766.22. Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991. 3:207–212.23. Lee JH, Heo SJ, Koak JY, Kim SK, Lee SJ, Lee SH. Cellular responses on anodized titanium discs after laser irradiation. Lasers Surg Med. 2008. 40:738–742.24. Aita H, Att W, Ueno T, Yamada M, Hori N, Iwasa F, Tsukimura N, Ogawa T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009. 5:3247–3257.25. Hori N, Ueno T, Suzuki T, Yamada M, Att W, Okada S, Ohno A, Aita H, Kimoto K, Ogawa T. Ultraviolet light treatment for the restoration of age-related degradation of titanium bioactivity. Int J Oral Maxillofac Implants. 2010. 25:49–62.26. Att W, Hori N, Takeuchi M, Ouyang J, Yang Y, Anpo M, Ogawa T. Time-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materials. Biomaterials. 2009. 30:5352–5363.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Wettability of titanium implants depending upon surface properties
- Surface analyses of titanium substrate modified by anodization and nanoscale Ca-P deposition
- Wettability and drug delivery of functionally graded nano-micro porous titanium surface
- Effects of various surface treatments for titanium on surface micro roughness, static wettability, fibronectin adsorption
- Surface characteristics and bioactivity of an anodized titanium surface