Tuberc Respir Dis.
2012 Apr;72(4):343-351.
Dual Inhibition of PI3K/Akt/mTOR Pathway and Role of Autophagy in Non-Small Cell Lung Cancer Cells
- Affiliations
-
- 1Division of Pulmonology, Department of Internal Medicine, Korea Cancer Center Hospital, Seoul, Korea. cheol@kcch.re.kr
Abstract
- BACKGROUND
The phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling axis has emerged as a novel target for cancer therapy. Agents that inhibit this pathway are currently under development for lung cancer treatment. In the present study, we have tested whether dual inhibition of PI3K/Akt/mTOR signaling can lead to enahnced antitumor effects. We have also examined the role of autophagy during this process.
METHODS
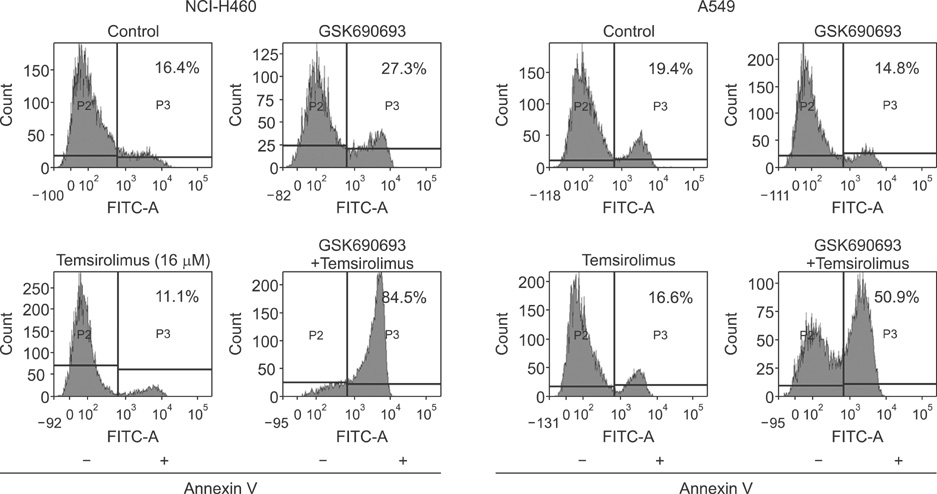
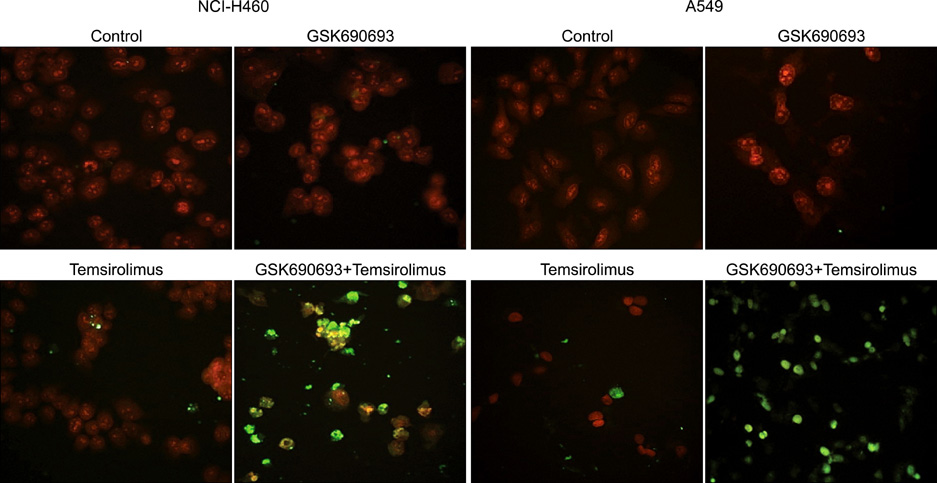
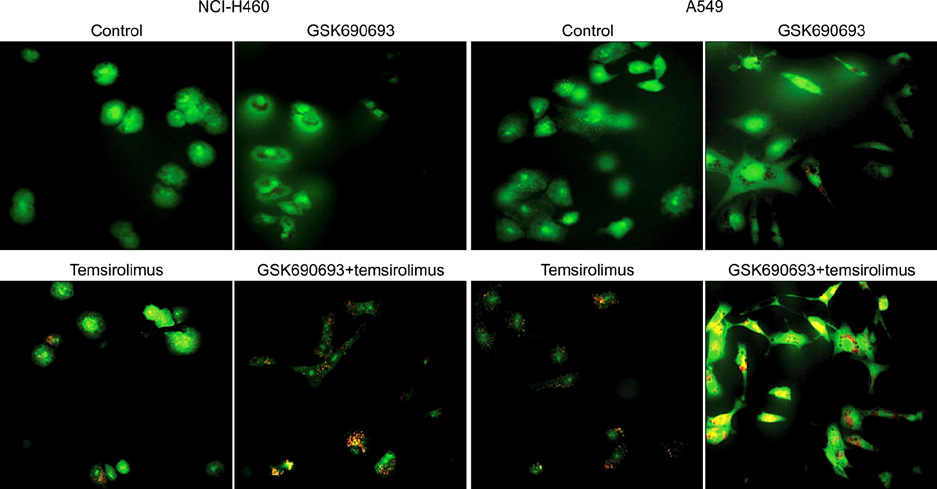
We analyzed the combination effect of the mTOR inhibitor, temsirolimus, and the Akt inhibitor, GSK690693, on the survival of NCI-H460 and A549 non-small cell lung cancer cells. Cell proliferation was determined by MTT assay and apoptosis induction was evaluated by flow cytometry and terminal deoxynucleotidyl transferase dUTP nick end labeling assay. Autophagy induction was also evaluated by acridine orange staining. Changes of apoptosis or autophagy-related proteins were evaluated by western blot analysis.
RESULTS
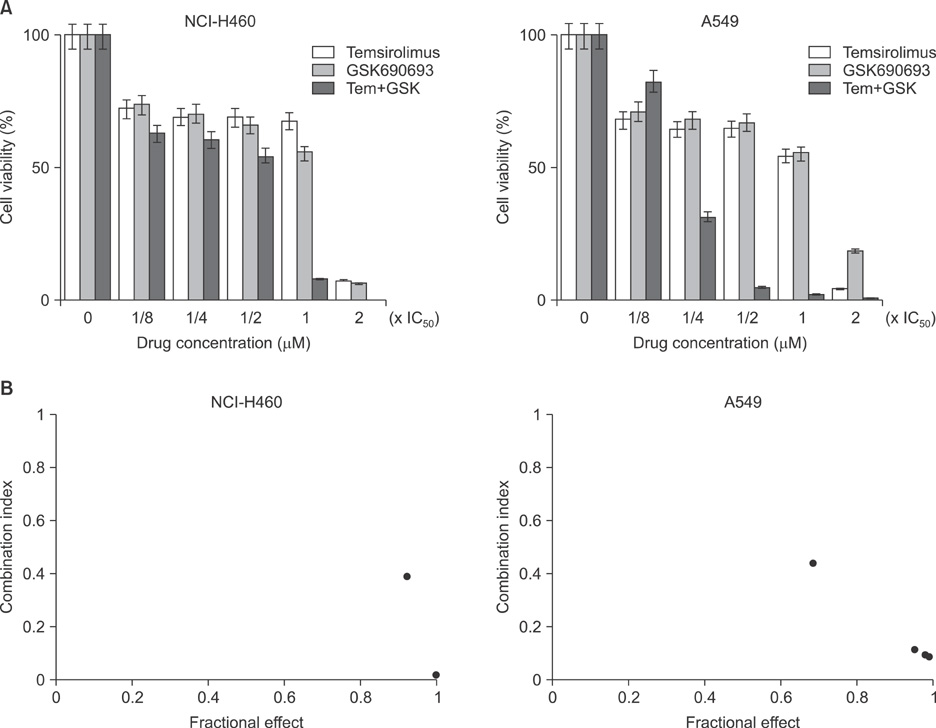
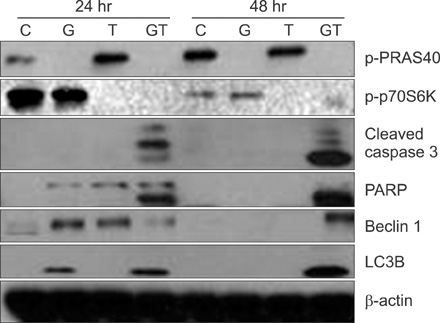
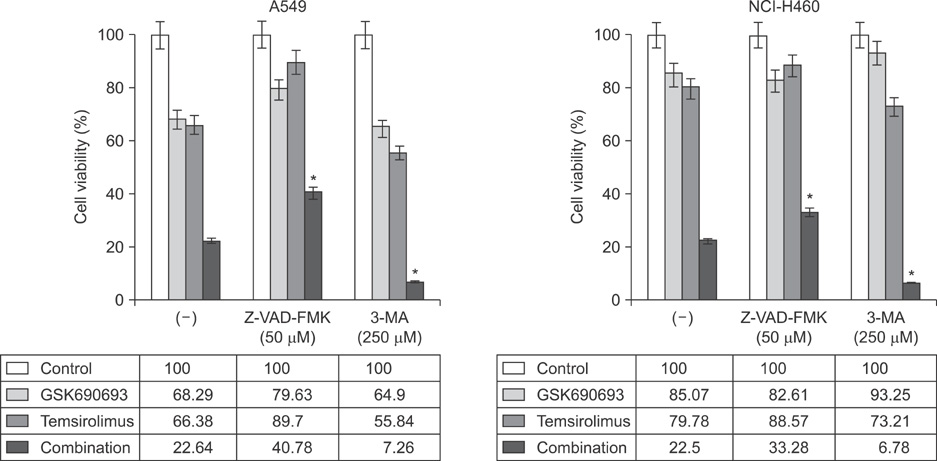
Combination treatment with temsirolimus and GSK690693 caused synergistically increased cell death in NCI-H460 and A549 cells. This was attributable to increased induction of apoptosis. Caspase 3 activation and poly(ADP-ribose) polymerase cleavage accompanied these findings. Autophagy also increased and inhibition of autophagy resulted in increased cell death, suggesting its cytoprotective role during this process.
CONCLUSION
Taken together, our results suggest that the combination of temsirolimus and GSK690693 could be a novel strategy for lung cancer therapy. Inhibition of autophagy could also be a promising method of enhancing the combination effect of these drugs.
Keyword
MeSH Terms
-
Acridine Orange
Apoptosis
Autophagy
Axis, Cervical Vertebra
Blotting, Western
Carcinoma, Non-Small-Cell Lung
Caspase 3
Cell Death
Cell Proliferation
DNA Nucleotidylexotransferase
Flow Cytometry
Lung Neoplasms
Oxadiazoles
Phosphatidylinositol 3-Kinases
Poly(ADP-ribose) Polymerases
Proteins
Sirolimus
TOR Serine-Threonine Kinases
Acridine Orange
Caspase 3
DNA Nucleotidylexotransferase
Oxadiazoles
Phosphatidylinositol 3-Kinases
Poly(ADP-ribose) Polymerases
Proteins
Sirolimus
TOR Serine-Threonine Kinases
Figure
Reference
-
1. Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998. 95:1432–1437.2. West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3'-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004. 64:446–451.3. Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer: is it becoming a reality? Nat Rev Clin Oncol. 2010. 7:401–414.4. Reungwetwattana T, Weroha SJ, Molina JR. Oncogenic pathways, molecularly targeted therapies, and high-lighted clinical trials in non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2011. 12. 06. [Epub]. http://dx.doi.org/10.1016/j.cllc.2011.09.004.5. Yamamoto H, Shigematsu H, Nomura M, Lockwood WW, Sato M, Okumura N, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008. 68:6913–6921.6. Kawano O, Sasaki H, Endo K, Suzuki E, Haneda H, Yukiue H, et al. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer. 2006. 54:209–215.7. Kawano O, Sasaki H, Okuda K, Yukiue H, Yokoyama T, Yano M, et al. PIK3CA gene amplification in Japanese non-small cell lung cancer. Lung Cancer. 2007. 58:159–160.8. Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007. 356:2271–2281.9. Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008. 372:449–456.10. Mita MM, Mita AC, Chu QS, Rowinsky EK, Fetterly GJ, Goldston M, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008. 26:361–367.11. Soria JC, Shepherd FA, Douillard JY, Wolf J, Giaccone G, Crino L, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol. 2009. 20:1674–1681.12. Molina JR, Mandrekar SJ, Rowland K, Reuter NF, Jett JR, Marks R, et al. A phase II NCCTG "window of opportunity front-line" study of the mTOR inhibitor, CCI-779 (temsirolimus) given as a single agent in patients with advanced NSCLC: D7-07. J Thorac Oncol. 2007. 2:S413.13. Ihle NT, Paine-Murrieta G, Berggren MI, Baker A, Tate WR, Wipf P, et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005. 4:1349–1357.14. Ihle NT, Williams R, Chow S, Chew W, Berggren MI, Paine-Murrieta G, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004. 3:763–772.15. Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008. 68:2366–2374.16. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007. 7:169–181.17. Arteaga CL. HER3 and mutant EGFR meet MET. Nat Med. 2007. 13:675–677.18. Janku F, Garrido-Laguna I, Petruzelka LB, Stewart DJ, Kurzrock R. Novel therapeutic targets in non-small cell lung cancer. J Thorac Oncol. 2011. 6:1601–1612.19. Marinov M, Fischer B, Arcaro A. Targeting mTOR signaling in lung cancer. Crit Rev Oncol Hematol. 2007. 63:172–182.20. Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol. 2009. 36:Suppl 3. S3–S17.21. Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Santos ED, et al. Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin Cancer Res. 2011. 17:3193–3203.22. Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011. 10:2426–2436.23. Xu CX, Li Y, Yue P, Owonikoko TK, Ramalingam SS, Khuri FR, et al. The combination of RAD001 and NVP-BEZ235 exerts synergistic anticancer activity against non-small cell lung cancer in vitro and in vivo. PLoS One. 2011. 6:e20899.24. Yuan J, Mehta PP, Yin MJ, Sun S, Zou A, Chen J, et al. PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity. Mol Cancer Ther. 2011. 10:2189–2199.25. Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev. 2011. 21:113–119.26. Maycotte P, Thorburn A. Autophagy and cancer therapy. Cancer Biol Ther. 2011. 11:127–137.27. Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? Autophagy. 2009. 5:442–450.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dasatinib induces apoptosis and autophagy by suppressing the PI3K/Akt/mTOR pathway in bladder cancer cells
- Delphinidin enhances radio-therapeutic effects via autophagyinduction and JNK/MAPK pathway activation in non-small celllung cancer
- Induction of Hepatocellular Carcinoma Cell Cycle Arrest and Apoptosis by Dendropanax morbifera Leveille Leaf Extract via the PI3K/AKT/mTOR Pathway
- Inhibition of DNMT3B and PI3K/AKT/mTOR and ERK Pathways as a Novel Mechanism of Volasertib on Hypomethylating Agent-Resistant Cells
- Extracellular Ubiquitin Enhances Autophagy and Inhibits Mitochondrial Apoptosis Pathway to Protect Neurons Against Spinal Cord Ischemic Injury via CXCR4