Korean J Urol.
2012 Aug;53(8):556-563.
Isolation and Characterization of Smooth Muscle Cells from Rat Corpus Cavernosum Tissue for the Study of Erectile Dysfunction
- Affiliations
-
- 1Department of Urology, Konkuk University School of Medicine, Chungju, Korea. yskurol@kku.ac.kr
- 2Department of Physiology, Konkuk University School of Medicine, Chungju, Korea.
- 3Department of Urology, Inha University School of Medicine, Incheon, Korea.
Abstract
- PURPOSE
Primary culture of the cavernous smooth muscle cells from corpus cavernous tissues is known to be difficult, mainly because of contamination with fibroblasts. We applied a new method for better isolation of rat penile smooth muscle cells (RPSMCs) from rat corpus cavernosum tissue for reliable ex vivo research on erectile dysfunction.
MATERIALS AND METHODS
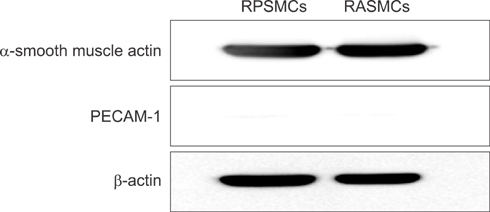
With the use of 8-week-old adult male Sprague-Dawley rats, ex vivo migrations of rat cavernous tissue were measured by penis and aortic ring assay by use of a Matrigel-based D-valine-modified culture method. The expression of alpha-smooth muscle actin (alpha-SMA) and platelet/endothelial cell adhesion molecule (PECAM)-1 in the RPSMCs was determined by standard immunofluorescent staining and immunoblotting. The expression patterns of phosphodiesterase (PDE) family mRNA in RPSMCs were compared with patterns in rat aortic smooth muscle cells (RASMCs) by use of quantitative real-time reverse transcription polymerase chain reaction.
RESULTS
Immunocytochemical staining showed greater alpha-SMA-positive and PCAM-1-negative fluorescence. Moreover, whereas the expression of alpha-SMA was detected in the RPSMCs, that of PECAM-1 was not. The levels of PDE1A, PDE1B, PDE1C, PDE2A, PDE3A, PDE4A, PDE4B, PDE4C, PDE4D, and PDE5A mRNA in the RPSMCs were about 3.2-, 4.4-, 3.4-, 29.0-, 3.5-, 2.8-, 2.9-, 6.1-, 45.0-, and 6.0-fold the corresponding expression in RASMCs.
CONCLUSIONS
We developed a two-stage tissue culture method utilizing a Matrigel-based sprouting culture system to facilitate stromal cell sprouting and an adherent culture system using D-valine to eliminate the contamination of fibroblasts into the smooth muscle cells.
Keyword
MeSH Terms
-
Actins
Adult
Animals
Antigens, CD31
Caves
Cell Adhesion
Collagen
Drug Combinations
Erectile Dysfunction
Fibroblasts
Fluorescence
Humans
Immunoblotting
Laminin
Male
Muscle, Smooth
Muscles
Myocytes, Smooth Muscle
Penile Erection
Penis
Primary Cell Culture
Proteoglycans
Rats
Rats, Sprague-Dawley
Reverse Transcription
RNA, Messenger
Stromal Cells
Actins
Antigens, CD31
Collagen
Drug Combinations
Laminin
Proteoglycans
RNA, Messenger
Figure
Reference
-
1. Martinez-Salamanca JI, Martinez-Ballesteros C, Portillo L, Gabancho S, Moncada I, Carballido J. Physiology of erection. Arch Esp Urol. 2010. 63:581–588.2. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989. 320:1025–1030.3. Andersson KE. Neurophysiology/pharmacology of erection. Int J Impot Res. 2001. 13:Suppl 3. S8–S17.4. Christ GJ, Hodges S. Molecular mechanisms of detrusor and corporal myocyte contraction: identifying targets for pharmacotherapy of bladder and erectile dysfunction. Br J Pharmacol. 2006. 147:Suppl 2. S41–S55.5. Chung H, Lee CK, Kim B, Kim HS, Kim TW, Paick SH, et al. Proteomic analysis of penile protein alterations in a rat model of cavernous nerve injury. Korean J Urol. 2009. 50:498–504.6. Koehler N, Holze S, Gansera L, Rebmann U, Roth S, Scholz HJ, et al. Erectile dysfunction after radical prostatectomy: the impact of nerve-sparing status and surgical approach. Int J Impot Res. 2012. 24:155–160.7. Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, et al. Hypercholesterolemia-induced erectile dysfunction: endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med. 2010. 7:3023–3032.8. Qiu X, Fandel TM, Lin G, Huang YC, Dai YT, Lue TF, et al. Cavernous smooth muscle hyperplasia in a rat model of hyperlipidaemia-associated erectile dysfunction. BJU Int. 2011. 108:1866–1872.9. Granchi S, Vannelli GB, Vignozzi L, Crescioli C, Ferruzzi P, Mancina R, et al. Expression and regulation of endothelin-1 and its receptors in human penile smooth muscle cells. Mol Hum Reprod. 2002. 8:1053–1064.10. Pilatz A, Schultheiss D, Gabouev AI, Schlote N, Mertsching H, Jonas U, et al. Isolation of primary endothelial and stromal cell cultures of the corpus cavernosum penis for basic research and tissue engineering. Eur Urol. 2005. 47:710–718.11. Benton G, George J, Kleinman HK, Arnaoutova IP. Advancing science and technology via 3D culture on basement membrane matrix. J Cell Physiol. 2009. 221:18–25.12. Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010. 10:1886–1890.13. Yin GN, Ryu JK, Kwon MH, Shin SH, Jin HR, Song KM, et al. Matrigel-based sprouting endothelial cell culture system from mouse corpus cavernosum is potentially useful for the study of endothelial and erectile dysfunction related to high-glucose exposure. J Sex Med. 2012. 9:1760–1772.14. Hongpaisan J. Inhibition of proliferation of contaminating fibroblasts by D-valine in cultures of smooth muscle cells from human myometrium. Cell Biol Int. 2000. 24:1–7.15. Lee CK, Han JS, Won KJ, Jung SH, Park HJ, Lee HM, et al. Diminished expression of dihydropteridine reductase is a potent biomarker for hypertensive vessels. Proteomics. 2009. 9:4851–4858.16. Lee CK, Park HJ, So HH, Kim HJ, Lee KS, Choi WS, et al. Proteomic profiling and identification of cofilin responding to oxidative stress in vascular smooth muscle. Proteomics. 2006. 6:6455–6475.17. Kimura M, Rabbani ZN, Zodda AR, Yan H, Jackson IL, Polascik TJ, et al. Role of oxidative stress in a rat model of radiation-induced erectile dysfunction. J Sex Med. 2012. 9:1535–1549.18. Ozden E, Ozturk B, Kosan M, Tezel GG, Aki FT, Gur S, et al. Effect of sildenafil citrate on penile weight and physiology of cavernous smooth muscle in a post-radical prostatectomy model of erectile dysfunction in rats. Urology. 2011. 77:761.e1–761.e7.19. Waldkirch ES, Uckert S, Sohn M, Kuczyk MA, Hedlund P. Rho kinase (ROK)-related proteins in human cavernous arteries: an immunohistochemical and functional approach. J Sex Med. 2012. 9:1337–1343.20. Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Müller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002. 72:221–229.21. Moreland RB, Hsieh G, Nakane M, Brioni JD. The biochemical and neurologic basis for the treatment of male erectile dysfunction. J Pharmacol Exp Ther. 2001. 296:225–234.22. Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010. 5:291–296.23. Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998. 159:2164–2171.24. Kuthe A, Wiedenroth A, Magert HJ, Uckert S, Forssmann WG, Stief CG, et al. Expression of different phosphodiesterase genes in human cavernous smooth muscle. J Urol. 2001. 165:280–283.25. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995. 75:725–748.26. Manganiello VC, Murata T, Taira M, Belfrage P, Degerman E. Diversity in cyclic nucleotide phosphodiesterase isoenzyme families. Arch Biochem Biophys. 1995. 322:1–13.27. Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000. 87:825–830.28. Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003. 93:280–291.29. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995. 75:725–748.30. Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology. 2010. 59:367–374.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in Corpus Cavernosum after Partial Bladder Outlet Obstruction in Rat
- Altered Contents of Smooth Muscle and Collagen in Corpus Cavernosum in Patients with Erectile Dysfunction
- The Expression of eNOS and ET-1 in Corpus Cavernosum in Male Rat with Partial Bladder Outlet Obstruction
- Local Effect of Psychotherapeutic Agents on Rabbit Penile Corpus Cavernosum
- The Role of Nitric Oxide in the Relaxation of Canine Corpus Cavernosum Smooth Muscle