Nutr Res Pract.
2015 Aug;9(4):343-349. 10.4162/nrp.2015.9.4.343.
Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway
- Affiliations
-
- 1Department of Animal Biotechnology and Resource, Sahmyook University, Hwarangro 815, Nowon-gu, Seoul 139-742, Korea. jeonwm@syu.ac.kr
- 2Institute of Fundamental Sciences, Massey University, Private Bag 11 222, Palmerston North, New Zealand.
- KMID: 2313849
- DOI: http://doi.org/10.4162/nrp.2015.9.4.343
Abstract
- BACKGROUND/OBJECTIVES
Fermentation of dietary fiber results in production of various short chain fatty acids in the colon. In particular, butyrate is reported to regulate the physical and functional integrity of the normal colonic mucosa by altering mucin gene expression or the number of goblet cells. The objective of this study was to investigate whether butyrate modulates mucin secretion in LS174T human colorectal cells, thereby influencing the adhesion of probiotics such as Lactobacillus and Bifidobacterium strains and subsequently inhibiting pathogenic bacteria such as E. coli. In addition, possible signaling pathways involved in mucin gene regulation induced by butyrate treatment were also investigated.
MATERIALS/METHODS
Mucin protein content assay and periodic acid-Schiff (PAS) staining were performed in LS174T cells treated with butyrate at various concentrations. Effects of butyrate on the ability of probiotics to adhere to LS174T cells and their competition with E. coli strains were examined. Real time polymerase chain reaction for mucin gene expression and Taqman array 96-well fast plate-based pathway analysis were performed on butyrate-treated LS174T cells.
RESULTS
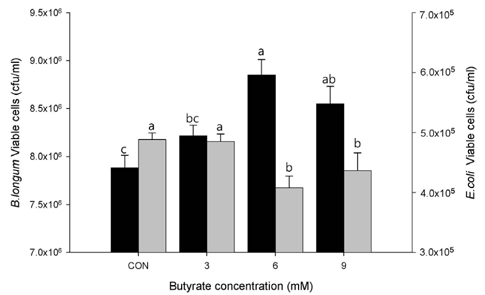
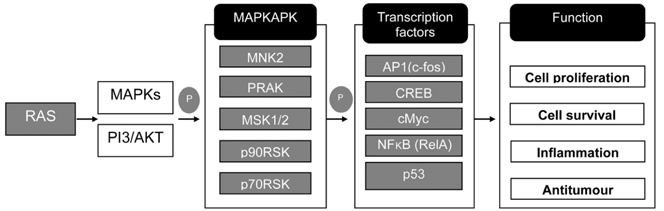
Treatment with butyrate resulted in a dose-dependent increase in mucin protein contents in LS174T cells with peak effects at 6 or 9 mM, which was further confirmed by PAS staining. Increase in mucin protein contents resulted in elevated adherence of probiotics, which subsequently reduced the adherent ability of E. coli. Treatment with butyrate also increased transcriptional levels of MUC3, MUC4, and MUC12, which was accompanied by higher gene expressions of signaling kinases and transcription factors involved in mitogen-activated protein kinase (MAPK) signaling pathways.
CONCLUSIONS
Based on our results, butyrate is an effective regulator of modulation of mucin protein production at the transcriptional and translational levels, resulting in changes in the adherence of gut microflora. Butyrate potentially stimulates the MAPK signaling pathway in intestinal cells, which is positively correlated with gut defense.
Keyword
MeSH Terms
-
Bacteria
Bifidobacterium
Butyrates*
Colon
Dietary Fiber
Fatty Acids
Fermentation
Gene Expression
Goblet Cells
Humans
Lactobacillus
Mucins*
Mucous Membrane
Phosphotransferases
Probiotics
Protein Kinases
Real-Time Polymerase Chain Reaction
Transcription Factors
Butyrates
Fatty Acids
Mucins
Phosphotransferases
Protein Kinases
Transcription Factors
Figure
Reference
-
1. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001; 81:1031–1064.
Article2. Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp Biochem Physiol A Mol Integr Physiol. 2000; 125:525–531.
Article3. Blottière HM, Buecher B, Galmiche JP, Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc Nutr Soc. 2003; 62:101–106.
Article4. Young GP, Hu Y, Le Leu RK, Nyskohus L. Dietary fibre and colorectal cancer: a model for environment--gene interactions. Mol Nutr Food Res. 2005; 49:571–584.
Article5. Olmo N, Turnay J, Pérez-Ramos P, Lecona E, Barrasa JI, López de Silanes I, Lizarbe MA. In vitro models for the study of the effect of butyrate on human colon adenocarcinoma cells. Toxicol In Vitro. 2007; 21:262–270.
Article6. Yang J, Kawai Y, Hanson RW, Arinze IJ. Sodium butyrate induces transcription from the G alpha(i2) gene promoter through multiple Sp1 sites in the promoter and by activating the MEK-ERK signal transduction pathway. J Biol Chem. 2001; 276:25742–25752.
Article7. Shah P, Nankova BB, Parab S, La Gamma EF. Short chain fatty acids induce TH gene expression via ERK-dependent phosphorylation of CREB protein. Brain Res. 2006; 1107:13–23.
Article8. Zhang Y, Zhou L, Bao YL, Wu Y, Yu CL, Huang YX, Sun Y, Zheng LH, Li YX. Butyrate induces cell apoptosis through activation of JNK MAP kinase pathway in human colon cancer RKO cells. Chem Biol Interact. 2010; 185:174–181.
Article9. Zuo L, Lu M, Zhou Q, Wei W, Wang Y. Butyrate suppresses proliferation and migration of RKO colon cancer cells though regulating endocan expression by MAPK signaling pathway. Food Chem Toxicol. 2013; 62:892–900.
Article10. Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000; 47:589–594.
Article11. Han KS, Deglaire A, Sengupta R, Moughan PJ. Hydrolyzed casein influences intestinal mucin gene expression in the rat. J Agric Food Chem. 2008; 56:5572–5576.
Article12. Allen A, Hutton DA, Pearson JP. The MUC2 gene product: a human intestinal mucin. Int J Biochem Cell Biol. 1998; 30:797–801.
Article13. Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004; 287:G1168–G1174.14. Trompette A, Blanchard C, Zoghbi S, Bara J, Claustre J, Jourdan G, Chayvialle JA, Plaisancé P. The DHE cell line as a model for studying rat gastro-intestinal mucin expression: effects of dexamethasone. Eur J Cell Biol. 2004; 83:347–358.
Article15. Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol. 2008; 10:37–54.16. Ouwehand AC, Salminen S. In vitro adhesion assays for probiotics and their in vivo relevance: a review. Microb Ecol Health Dis. 2003; 15:175–184.
Article17. Valeriano VD, Parungao-Balolong MM, Kang DK. In vitro evaluation of the mucin-adhesion ability and probiotic potential of Lactobacillus mucosae LM1. J Appl Microbiol. 2014; 117:485–497.
Article18. Bucki R, Namiot DB, Namiot Z, Savage PB, Janmey PA. Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J Antimicrob Chemother. 2008; 62:329–335.
Article19. Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010; 12:319–330.
Article20. Kober OI, Ahl D, Pin C, Holm L, Carding SR, Juge N. gammadelta T-cell-deficient mice show alterations in mucin expression, glycosylation, and goblet cells but maintain an intact mucus layer. Am J Physiol Gastrointest Liver Physiol. 2014; 306:G582–G593.21. Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007; 102:1197–1208.
Article22. Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002; 217:133–139.
Article23. Gaudier E, Rival M, Buisine MP, Robineau I, Hoebler C. Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol Res. 2009; 58:111–119.
Article24. Hatayama H, Iwashita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Commun. 2007; 356:599–603.
Article25. Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000; 46:218–224.
Article26. Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA. Cell signaling through membrane mucins. Bioessays. 2003; 25:66–71.
Article27. Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg Infect Dis. 2005; 11:603–609.28. Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001; 73:1131S–1141S.
Article29. Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003; 52:827–833.
Article30. Fujiwara S, Hashiba H, Hirota T, Forstner JF. Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide. Appl Environ Microbiol. 1997; 63:506–512.
Article31. Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002; 18:586–590.
Article32. Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001; 93:1062–1074.
Article33. Wu KL, Huang EY, Jhu EW, Huang YH, Su WH, Chuang PC, Yang KD. Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway. J Gastroenterol. 2013; 48:350–359.
Article34. Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004; 68:320–344.
Article35. Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005; 123:569–580.
Article36. Reber L, Vermeulen L, Haegeman G, Frossard N. Ser276 phosphorylation of NF-κB p65 by MSK1 controls SCF expression in inflammation. PLoS One. 2009; 4:e4393.37. Gancz D, Lusthaus M, Fishelson Z. A role for the NF-kappaB pathway in cell protection from complement-dependent cytotoxicity. J Immunol. 2012; 189:860–866.
Article38. Daniel P, Filiz G, Brown DV, Hollande F, Gonzales M, D'Abaco G, Papalexis N, Phillips WA, Malaterre J, Ramsay RG, Mantamadiotis T. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis. 2014; 3:e108.
Article39. Lane DP. Cancer. p53, guardian of the genome. Nature. 1992; 358:15–16.40. Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007; 26:1306–1316.
Article41. Williams EA, Coxhead JM, Mathers JC. Anti-cancer effects of butyrate: use of micro-array technology to investigate mechanisms. Proc Nutr Soc. 2003; 62:107–115.
Article42. Wang HG, Huang XD, Shen P, Li LR, Xue HT, Ji GZ. Anticancer effects of sodium butyrate on hepatocellular carcinoma cells in vitro. Int J Mol Med. 2013; 31:967–974.
Article43. Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem. 2002; 277:32258–32267.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ras Mitogen-activated Protein Kinase Signaling and Kinase Suppressor of Ras as Therapeutic Targets for Hepatocellular Carcinoma
- Effect of Polyinosinic-Polycytidylic Acid on MUC5B Expression in Human Airway Epithelial Cells
- Kaempferol Regulates the Expression of Airway MUC5AC Mucin Gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 Signaling Pathways
- Phorbol 12-Myristate 13-Acetate Induces MUC16 Expression via PKCdelta and p38 in Human Airway Epithelial Cells
- Butyrate regulates leptin expression through different signaling pathways in adipocytes