Nutr Res Pract.
2014 Oct;8(5):487-493. 10.4162/nrp.2014.8.5.487.
D. candidum has in vitro anticancer effects in HCT-116 cancer cells and exerts in vivo anti-metastatic effects in mice
- Affiliations
-
- 1College of Food Science, Southwest University, 2 Tiansheng Road, Beibei District, Chongqing 400067, China. birget@swu.edu.cn
- 2Department of Biological and Chemical Engineering, Chongqing University of Education, China.
- KMID: 2313768
- DOI: http://doi.org/10.4162/nrp.2014.8.5.487
Abstract
- BACKGROUND/OBJECTIVES
D. candidum is a traditional Chinese food or medicine widely used in Asia. There has been little research into the anticancer effects of D. candidum, particularly the effects in colon cancer cells. The aim of this study was to investigate the anticancer effects of D. candidum in vitro and in vivo.
MATERIALS/METHODS
The in vitro anti-cancer effects on HCT-116 colon cancer cells and in vivo anti-metastatic effects of DCME (Dendrobium canidum methanolic extract) were examined using the experimental methods of MTT assay, DAPI staining, flow cytometry analysis, RT-PCR, and Western blot analysis.
RESULTS
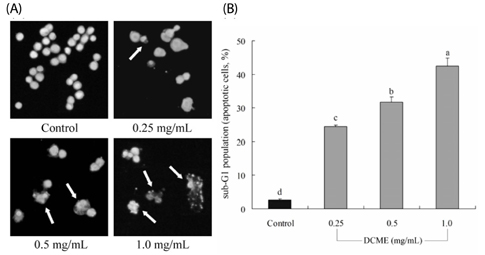
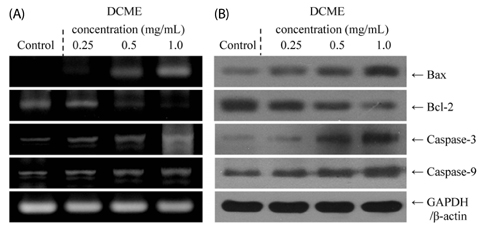
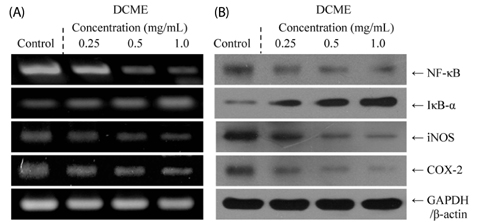
At a concentration of 1.0 mg/mL, DCME inhibited the growth of HCT-116 cells by 84%, which was higher than at concentrations of 0.5 and 0.25 mg/mL. Chromatin condensation and formation of apoptotic bodies were observed in cancer cells cultured with DCME as well. In addition, DCME induced significant apoptosis in cancer cells by upregulation of Bax, caspase 9, and caspase 3, and downregulation of Bcl-2. Expression of genes commonly associated with inflammation, NF-kappaB, iNOS, and COX-2, was significantly downregulated by DCME. DCME also exerted an anti-metastasis effect on cancer cells as demonstrated by decreased expression of MMP genes and increased expression of TIMPs, which was confirmed by the inhibition of induced tumor metastasis in colon 26-M3.1 cells in BALB/c mice.
CONCLUSIONS
Our results demonstrated that D. candidum had a potent in vitro anti-cancer effect, induced apoptosis, exhibited anti-inflammatory activities, and exerted in vivo anti-metastatic effects.
MeSH Terms
Figure
Reference
-
1. Jones WE, Kuehnle AR, Arumuganathan K. Nuclear DNA content of 26 orchid (Orchidaceae) genera with emphasis on Dendrobium. Ann Bot. 1998; 82:189–194.
Article2. Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004; 320:1103–1111.
Article3. Shao H, Zhang LQ, Li JM, Wei RC. Advances in research of Dendrobium officinale. Chin Tradit Herb Drugs. 2004; 35:109–112.4. Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000; 21:485–495.
Article5. Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001; 107:7–11.
Article6. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477:267–283.
Article7. Bao LJ, Wang JH, Luo JP. Inhibitory effects of water extracts from four species of Dendrobiums on HelaS3 cells and HepG2 cells. J Anhui Agric Sci. 2008; 36:15968–15970.8. Jung KO, Park SY, Park KY. Longer aging time increases the anticancer and antimetastatic properties of doenjang. Nutrition. 2006; 22:539–545.
Article9. van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011; 731:237–245.
Article10. Harish Kumar G, Vidya Priyadarsini R, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2010; 28:392–401.
Article11. Malla R, Gopinath S, Alapati K, Gondi CS, Gujrati M, Dinh DH, Mohanam S, Rao JS. Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/Akt pathway in gliomas. PLoS One. 2010; 5:e13731.
Article12. Li Y, Wang C, Wang F, Dong H, Guo S, Yang J, Xiao P. Chemical constituents of Dendrobium candidum. Zhongguo Zhong Yao Za Zhi. 2010; 35:1715–1719.13. Li J, Li S, Huang D, Zhao X, Cai G. Advances in the of resources, constituents and pharmacological effect of Dendrobium officinale. Ke Ji Dao Bao. 2011; 29:74–79.14. Milanezi F, Leitão D, Ricardo S, Augusto I, Schmitt F. Evaluation of HER2 in breast cancer: reality and expectations. Expert Opin Med Diagn. 2009; 3:607–620.
Article15. Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998; 16:395–419.
Article16. Kidd VJ. Proteolytic activities that mediate apoptosis. Annu Rev Physiol. 1998; 60:533–573.
Article17. Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999; 274:21155–21161.
Article18. Delić R, Štefanović M. Optimal laboratory panel for predicting preeclampsia. J Matern Fetal Neonatal Med. 2010; 23:96–102.
Article19. Baeuerle PA. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998; 95:729–731.20. Sánchez-Pérez I, Benitah SA, Martínez-Gomariz M, Lacal JC, Perona R. Cell stress and MEKK1-mediated c-Jun activation modulate NFkappaB activity and cell viability. Mol Biol Cell. 2002; 13:2933–2945.
Article21. Klein CA. Cancer. The metastasis cascade. Science. 2008; 321:1785–1787.22. Ha ES, Hwang SH, Shin KS, Yu KW, Lee KH, Choi JS, Park WM, Yoon TJ. Anti-metastatic activity of glycoprotein fractionated from Acanthopanax senticosus, involvement of NK-cell and macrophage activation. Arch Pharm Res. 2004; 27:217–224.
Article23. Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004; 23:101–117.
Article24. Mysliwiec AG, Ornstein DL. Matrix metalloproteinases in colorectal cancer. Clin Colorectal Cancer. 2002; 1:208–219.
Article25. Krüger A, Sanchez-Sweatman OH, Martin DC, Fata JE, Ho AT, Orr FW, Rüther U, Khokha R. Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene. 1998; 16:2419–2423.
Article26. Shin DY, Kim GY, Kim JI, Yoon MK, Kwon TK, Lee SJ, Choi YW, Kang HS, Yoo YH, Choi YH. Anti-invasive activity of diallyl disulfide through tightening of tight junctions and inhibition of matrix metalloproteinase activities in LNCaP prostate cancer cells. Toxicol In Vitro. 2010; 24:1569–1576.
Article27. Wang Q, Sun P, Li G, Zhu K, Wang C, Zhao X. Inhibitory effects of Wall ex Lindl. on azoxymethane- and dextran sulfate sodium-induced colon carcinogenesis in C57BL/6 mice. Oncol Lett. 2014; 7:493–498.
Article28. Wanqian L, Bochu W, Chuanren D, Biao L. A method for isolating functional RNA from callus of Dendrobium candidum contented rich polysaccharides. Colloids Surf B Biointerfaces. 2005; 42:259–262.
Article29. Chen J, Qi H, Li JB, Yi YQ, Chen D, Hu XH, Wang ML, Sun XL, Wei XY. Experimental study on Dendrobium candidum polysaccharides on promotion of hair growth. Zhongguo Zhong Yao Za Zhi. 2014; 39:291–295.30. Gao J, Jin R, Wu Y, Zhang H, Zhang D, Chang Y, Hu Z. Comparative study of tissue cultured Dendrobium protocorm with natural Dendrobium candidum on immunological function. Zhong Yao Cai. 2002; 25:487–489.31. Chaotham C, Pongrakhananon V, Sritularak B, Chanvorachote P. A Bibenzyl from Dendrobium ellipsophyllum inhibits epithelial-to-mesenchymal transition and sensitizes lung cancer cells to anoikis. Anticancer Res. 2014; 34:1931–1938.32. Deng MZ, Li TM. The effect of Dendrobium mixture on MDA, SOD, LPO and the immune function of aged rats. China J Chin Med. 2012; 27:58–59.33. Li GJ, Sun P, Qian Y, Zhao X. Preventive effect of lung metastases of Dendrobium candidum Wall ex Lindl. in BALB/c mice bearing 26-M3.1 cells. Oncol Lett. Forthcoming 2014.34. Chen J, Zhang LC, Xing YM, Wang YQ, Xing XK, Zhang DW, Liang HQ, Guo SX. Diversity and taxonomy of endophytic xylariaceous fungi from medicinal plants of Dendrobium (Orchidaceae). PLoS One. 2013; 8:e58268.
Article35. Prasad R, Koch B. Antitumor activity of ethanolic extract of Dendrobium formosum in T-cell lymphoma: an in vitro and in vivo study. Biomed Res Int. 2014; 2014:753451.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Genistein and Daidzein on the Growth of Human Colon Cancer HCT-116 Cells
- Anticancer Effect of Persimmon Leaf Extracts on Korean Gastric Cancer Cell
- Evaluation of the effects of disulfiram, an alcohol-aversive agent with anti-cancer activity, on mouse bone marrow cells
- Sanghuangporus sanghuang extract inhibits the proliferation and invasion of lung cancer cells in vitro and in vivo
- Umami taste receptor suppresses cancer cachexia by regulating skeletal muscle atrophy in vivo and in vitro