Korean J Physiol Pharmacol.
2022 May;26(3):157-164. 10.4196/kjpp.2022.26.3.157.
Evaluation of the effects of disulfiram, an alcohol-aversive agent with anti-cancer activity, on mouse bone marrow cells
- Affiliations
-
- 1College of Veterinary Medicine, Jeju National University, Jeju 63243, Korea
- 2Veterinary Medical Research Institute, Jeju National University, Jeju 63243, Korea
- KMID: 2529398
- DOI: http://doi.org/10.4196/kjpp.2022.26.3.157
Abstract
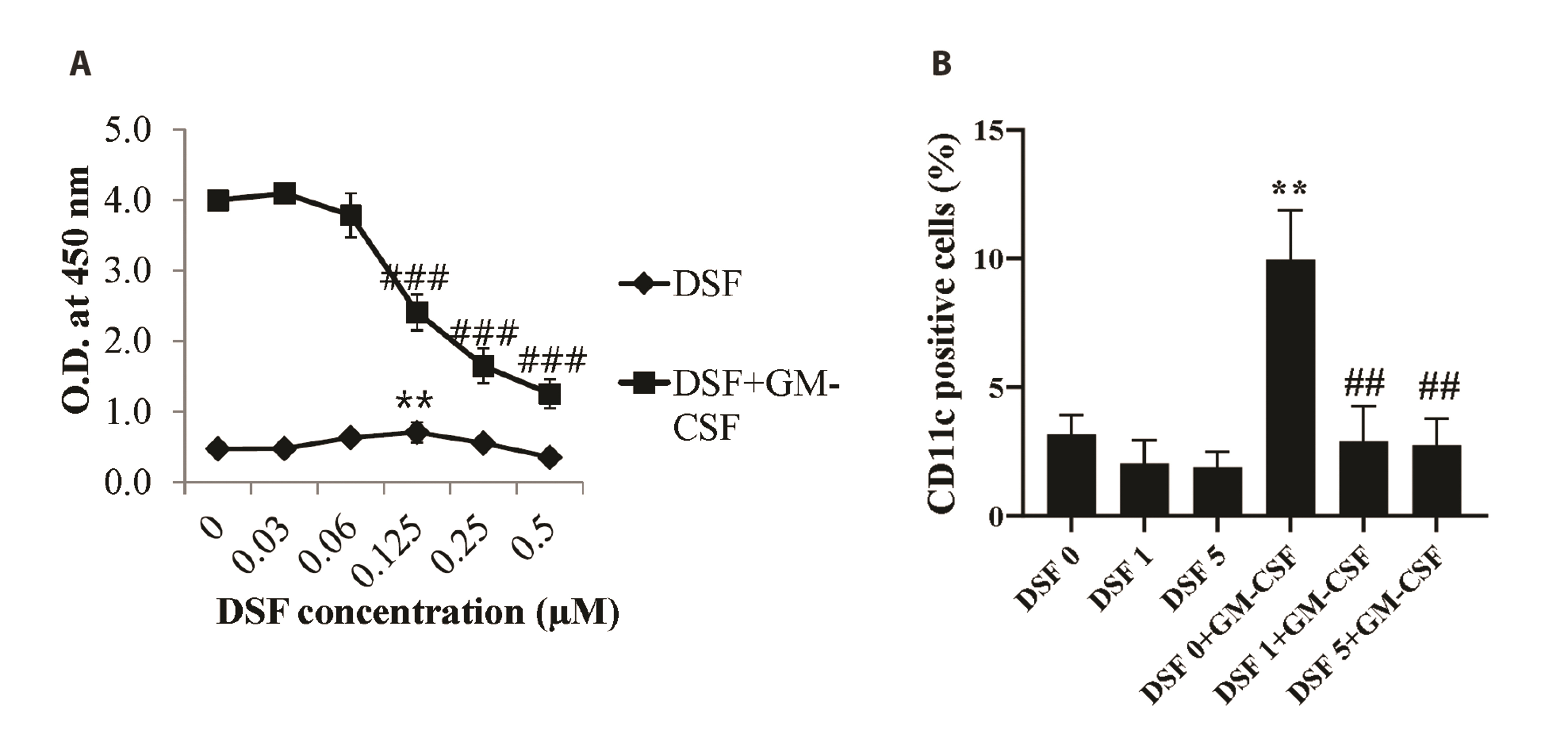
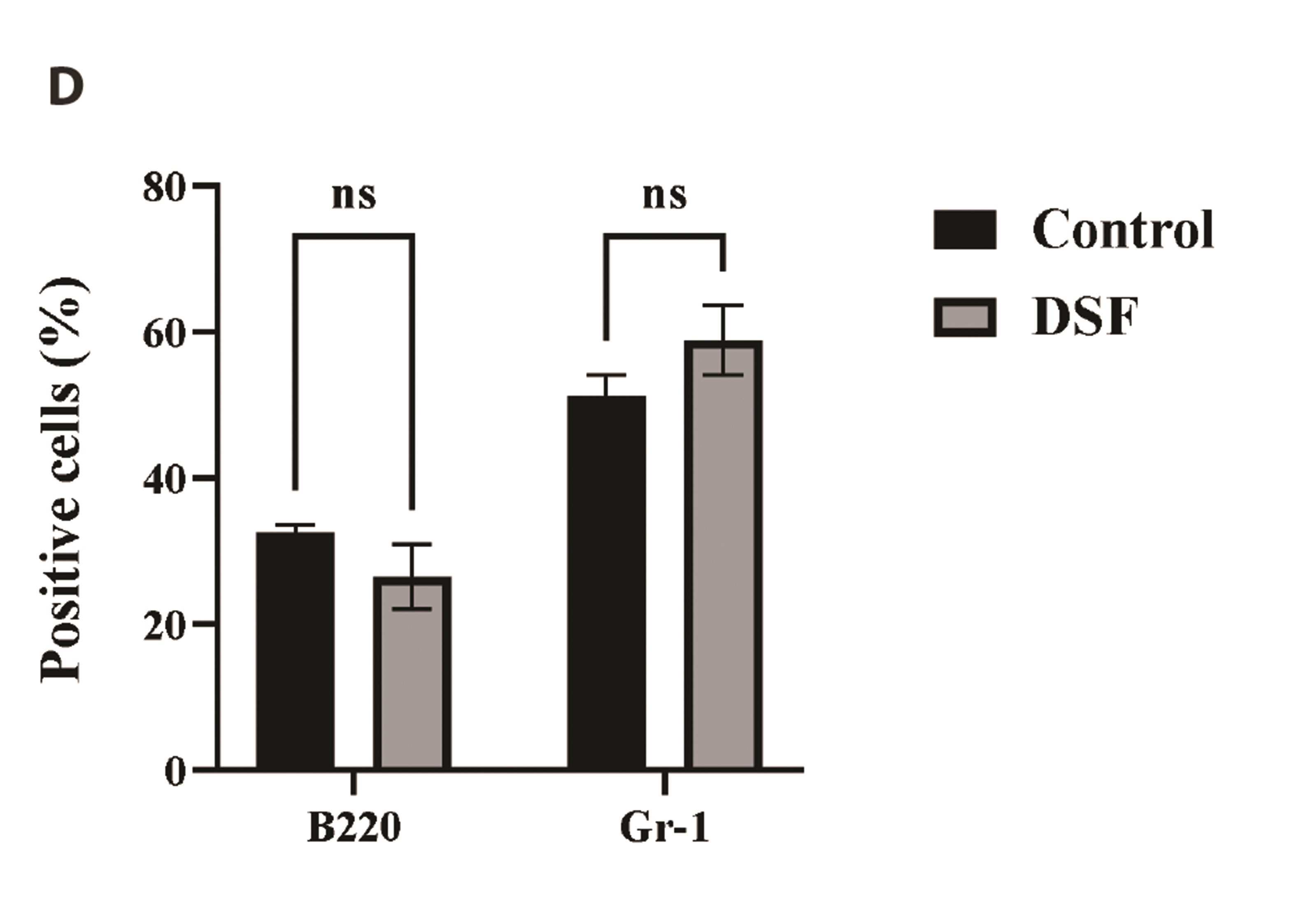
- Disulfiram (DSF) is an aldehyde dehydrogenase inhibitor. DSF has potent anti-cancer activity for solid and hematological malignancies. Although the effects on cancer cells have been proven, there have been few studies on DSF toxicity in bone marrow cells (BMs). DSF reduces the metabolic activity and the mitochondrial membrane potential of BMs. In subset analyses, we confirmed that DSF does not affect the proportion of BMs. In addition, DSF significantly impaired the metabolic activity and differentiation of BMs treated with granulocyte macrophage-colony stimulating factor, an essential growth and differentiation factor for BMs. To measure DSF toxicity in BMs in vivo, mice were injected with 50 mg/kg, a dose used for anti-cancer effects. DSF did not significantly induce BM toxicity in mice and may be tolerated by antioxidant defense mechanisms. This is the first study on the effects of DSF on BMs in vitro and in vivo. DSF has been widely studied as an anti-cancer drug candidate, and many anti-cancer drugs lead to myelosuppression. In this regard, this study can provide useful information to basic science and clinical researchers.
Keyword
Figure
Reference
-
1. Mutschler J, Grosshans M, Soyka M, Rösner S. 2016; Current findings and mechanisms of action of disulfiram in the treatment of alcohol dependence. Pharmacopsychiatry. 49:137–141. DOI: 10.1055/s-0042-103592. PMID: 26987743.
Article2. Petersen EN. 1992; The pharmacology and toxicology of disulfiram and its metabolites. Acta Psychiatr Scand Suppl. 369:7–13. DOI: 10.1111/j.1600-0447.1992.tb03309.x. PMID: 1471556.
Article3. O'Farrell TJ, Allen JP, Litten RZ. 1995; Disulfiram (antabuse) contracts in treatment of alcoholism. NIDA Res Monogr. 150:65–91. DOI: 10.1037/e495792006-007. PMID: 8742773.4. Viola-Rhenals M, Patel KR, Jaimes-Santamaria L, Wu G, Liu J, Dou QP. 2018; Recent advances in antabuse (Disulfiram): the importance of its metal-binding ability to its anticancer activity. Curr Med Chem. 25:506–524. DOI: 10.2174/0929867324666171023161121. PMID: 29065820. PMCID: PMC6873226.
Article5. Jiao Y, Hannafon BN, Ding WQ. 2016; Disulfiram's anticancer activity: evidence and mechanisms. Anticancer Agents Med Chem. 16:1378–1384. DOI: 10.2174/1871520615666160504095040. PMID: 27141876.
Article6. Maxwell MB, Maher KE. 1992; Chemotherapy-induced myelosuppression. Semin Oncol Nurs. 8:113–123. DOI: 10.1016/0749-2081(92)90027-Z. PMID: 1621002.
Article7. Kwon MJ, Joo HG. 2018; Dapsone modulates lipopolysaccharide-activated bone marrow cells by inducing cell death and down-regulating tumor necrosis factor-α production. J Vet Sci. 19:744–749. DOI: 10.4142/jvs.2018.19.6.744. PMID: 30304888. PMCID: PMC6265590.
Article8. Lee YJ, Han Y, Joo HG. 2020; Bordetella bronchiseptica is a potent and safe adjuvant that enhances the antigen-presenting capability of dendritic cells. Korean J Physiol Pharmacol. 24:47–52. DOI: 10.4196/kjpp.2020.24.1.47. PMID: 31908574. PMCID: PMC6940501.
Article9. Ly JD, Grubb DR, Lawen A. 2003; The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 8:115–128. DOI: 10.1023/A:1022945107762. PMID: 12766472.10. Becher B, Tugues S, Greter M. 2016; GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity. 45:963–973. DOI: 10.1016/j.immuni.2016.10.026. PMID: 27851925.
Article11. Yang Z, Guo F, Albers AE, Sehouli J, Kaufmann AM. 2019; Disulfiram modulates ROS accumulation and overcomes synergistically cisplatin resistance in breast cancer cell lines. Biomed Pharmacother. 113:108727. DOI: 10.1016/j.biopha.2019.108727. PMID: 30870721.
Article12. Iljin K, Ketola K, Vainio P, Halonen P, Kohonen P, Fey V, Grafström RC, Perälä M, Kallioniemi O. 2009; High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin Cancer Res. 15:6070–6078. DOI: 10.1158/1078-0432.CCR-09-1035. PMID: 19789329.
Article13. Coffman RL, Weissman IL. 1981; B220: a B cell-specific member of th T200 glycoprotein family. Nature. 289:681–683. DOI: 10.1038/289681a0. PMID: 6970340.
Article14. Fleming TJ, Fleming ML, Malek TR. 1993; Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 151:2399–2408. PMID: 8360469.15. Hassani S, Ghaffari P, Chahardouli B, Alimoghaddam K, Ghavamzadeh A, Alizadeh S, Ghaffari SH. 2018; Disulfiram/copper causes ROS levels alteration, cell cycle inhibition, and apoptosis in acute myeloid leukaemia cell lines with modulation in the expression of related genes. Biomed Pharmacother. 99:561–569. DOI: 10.1016/j.biopha.2018.01.109. PMID: 29902866.
Article16. Yasui K, Kobayashi N, Yamazaki T, Agematsu K, Matsuzaki S, Ito S, Nakata S, Baba A, Koike K. 2005; Superoxide dismutase (SOD) as a potential inhibitory mediator of inflammation via neutrophil apoptosis. Free Radic Res. 39:755–762. DOI: 10.1080/10715760500104066. PMID: 16036355.
Article17. Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, Reis e Sousa C. 2015; GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c+MHCII+ macrophages and dendritic cells. Immunity. 42:1197–1211. DOI: 10.1016/j.immuni.2015.05.018. PMID: 26084029.
Article18. Skrott Z, Mistrik M, Andersen KK, Friis S, Majera D, Gursky J, Ozdian T, Bartkova J, Turi Z, Moudry P, Kraus M, Michalova M, Vaclavkova J, Dzubak P, Vrobel I, Pouckova P, Sedlacek J, Miklovicova A, Kutt A, Li J, et al. 2017; Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature. 552:194–199. DOI: 10.1038/nature25016. PMID: 29211715. PMCID: PMC5730499.
Article19. Bu W, Wang Z, Meng L, Li X, Liu X, Chen Y, Xin Y, Li B, Sun H. 2019; Disulfiram inhibits epithelial-mesenchymal transition through TGFβ-ERK-Snail pathway independently of Smad4 to decrease oral squamous cell carcinoma metastasis. Cancer Manag Res. 11:3887–3898. Erratum. DOI: 10.2147/CMAR.S348759. PMID: 34858055. PMCID: PMC8630542.20. Xu X, Xu J, Zhao C, Hou X, Li M, Wang L, Chen L, Chen Y, Zhu L, Yang H. 2019; Antitumor effects of disulfiram/copper complex in the poorly-differentiated nasopharyngeal carcinoma cells via activating ClC-3 chloride channel. Biomed Pharmacother. 120:109529. DOI: 10.1016/j.biopha.2019.109529. PMID: 31606620.
Article21. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. 2006; Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 160:1–40. DOI: 10.1016/j.cbi.2005.12.009. PMID: 16430879.
Article22. Niki E. 2010; Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. 49:503–515. DOI: 10.1016/j.freeradbiomed.2010.04.016. PMID: 20416370.23. Salem K, McCormick ML, Wendlandt E, Zhan F, Goel A. 2015; Copper-zinc superoxide dismutase-mediated redox regulation of bortezomib resistance in multiple myeloma. Redox Biol. 4:23–33. DOI: 10.1016/j.redox.2014.11.002. PMID: 25485927. PMCID: PMC4309843.
Article24. Madrigal-Bujaidar E, Velazquez-Guadarrama N, Morales-Ramirez P, Mendiola MT, Martínez AL, Chamorro G. 1999; Sister-chromatid exchanges induced by disulfiram in bone marrow and spermatogonial cells of mice treated in vivo. Food Chem Toxicol. 37:757–763. DOI: 10.1016/S0278-6915(99)00061-7. PMID: 10496378.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effects of fenbendazole, an anthelmintics, on lipopolysaccharide-activated mouse bone marrow cells
- Epileptic Seizure Due to Disulfiram Treatment
- Synergistic anticancer activity of disulfiram/copper against mouse lymphoma cells
- Hypotension Caused by a Disulfiram-Alcohol Reaction
- Anti-tumor activity and mitochondrial stability of disulfiram in HL-60 cells