Nutr Res Pract.
2014 Apr;8(2):138-145.
Antioxidative effects of fermented sesame sauce against hydrogen peroxide-induced oxidative damage in LLC-PK1 porcine renal tubule cells
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, Busan 609-735, Korea. kunypark@pusan.ac.kr
- 2Kimchi Research Institute, Pusan National University, 2, Busandaehak-ro 63 Beon-gil, Geumjeong, Busan 609-735, Korea.
- 3Daesang R&D, Icheon, Gyeonggi 467-813, Korea.
Abstract
- BACKGROUND/OBJECTIVES
This study was performed to investigate the in vitro antioxidant and cytoprotective effects of fermented sesame sauce (FSeS) against hydrogen peroxide (H2O2)-induced oxidative damage in renal proximal tubule LLC-PK1 cells.
MATERIALS/METHODS
1,1-diphenyl-2-picrylhydrazyl (DPPH), hydroxyl radical (*OH), and H2O2 scavenging assay was used to evaluate the in vitro antioxidant activity of FSeS. To investigate the cytoprotective effect of FSeS against H2O2-induced oxidative damage in LLC-PK1 cells, the cellular levels of reactive oxygen species (ROS), lipid peroxidation, and endogenous antioxidant enzymes including catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-px) were measured.
RESULTS
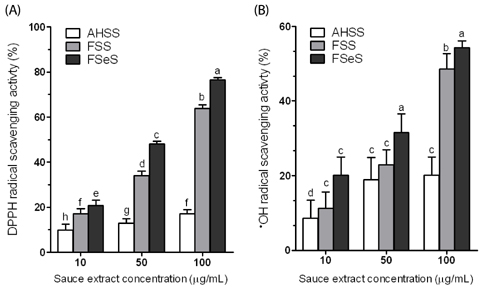
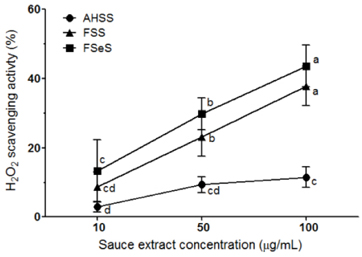
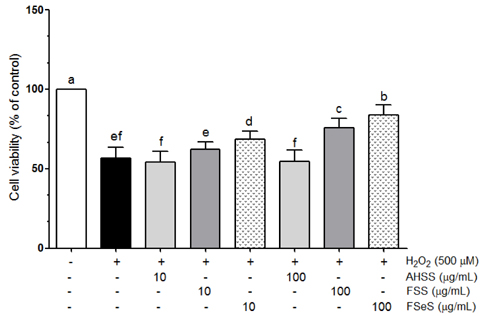
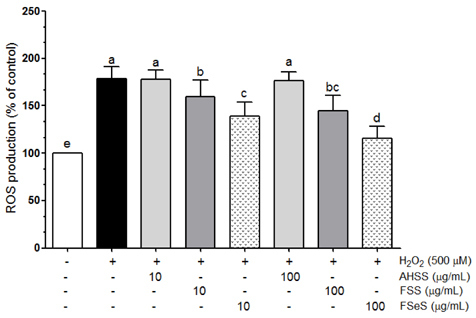
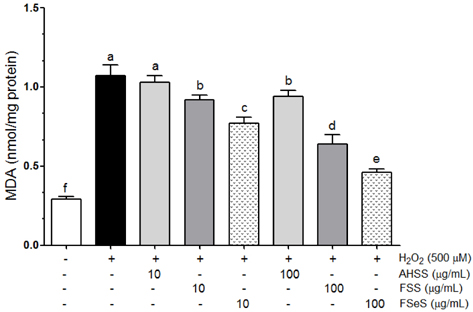
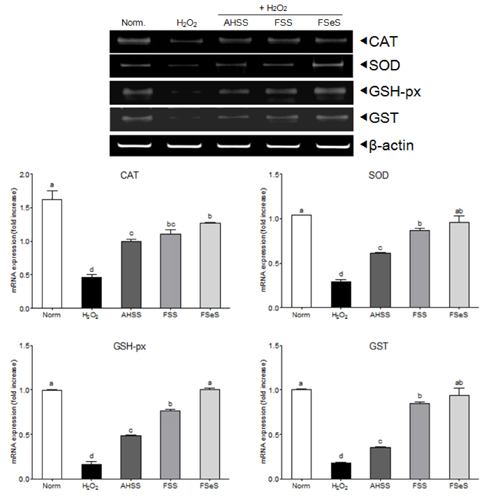
The ability of FSeS to scavenge DPPH, *OH and H2O2 was greater than that of FSS and AHSS. FSeS also significantly inhibited H2O2-induced (500 microM) oxidative damage in the LLC-PK1 cells compared to FSS and AHSS (P < 0.05). Following treatment with 100 microg/mL of FSeS and FSS to prevent H2O2-induced oxidation, cell viability increased from 56.7% (control) to 83.7% and 75.6%, respectively. However, AHSS was not able to reduce H2O2-induced cell damage (viability of the AHSS-treated cells was 54.6%). FSeS more effectively suppressed H2O2-induced ROS generation and lipid peroxidation compared to FSS and AHSS (P < 0.05). Compared to the other sauces, FSeS also significantly increased cellular CAT, SOD, and GSH-px activities and mRNA expression (P < 0.05). CONCULUSIONS: These results from the present study suggest that FSeS is an effective radical scavenger and protects against H2O2-induced oxidative damage in LLC-PK1 cells by reducing ROS levels, inhibiting lipid peroxidation, and stimulating antioxidant enzyme activity.
MeSH Terms
-
Animals
Catalase
Cats
Cell Survival
Glutathione Peroxidase
Hydrogen Peroxide
Hydrogen*
Hydroxyl Radical
Lipid Peroxidation
LLC-PK1 Cells
Oxidative Stress
Reactive Oxygen Species
RNA, Messenger
Sesamum*
Superoxide Dismutase
Swine
Catalase
Glutathione Peroxidase
Hydrogen
Hydrogen Peroxide
Hydroxyl Radical
RNA, Messenger
Reactive Oxygen Species
Superoxide Dismutase
Figure
Reference
-
1. Long LH, Kwee DC, Halliwell B. The antioxidant activities of seasonings used in Asian cooking. Powerful antioxidant activity of dark soy sauce revealed using the ABTS assay. Free Radic Res. 2000; 32:181–186.
Article2. Wang H, Jenner AM, Lee CY, Shui G, Tang SY, Whiteman M, Wenk MR, Halliwell B. The identification of antioxidants in dark soy sauce. Free Radic Res. 2007; 41:479–488.
Article3. Yang B, Yang H, Li J, Li Z, Jiang Y. Amino acid composition, molecular weight distribution and antioxidant activity of protein hydrolysates of soy sauce lees. Food Chem. 2011; 124:551–555.
Article4. Benjamin H, Storkson J, Nagahara A, Pariza MW. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by dietary soy sauce. Cancer Res. 1991; 51:2940–2942.5. Ito A, Watanabe H, Basaran N. Effects of soy products in reducing risk of spontaneous and neutron-induced liver-tumors in mice. Int J Oncol. 1993; 2:773–776.
Article6. Nagahara A, Benjamin H, Storkson J, Krewson J, Sheng K, Liu W, Pariza MW. Inhibition of benzo[a]pyrene-induced mouse forestomach neoplasia by a principal flavor component of Japanese-style fermented soy sauce. Cancer Res. 1992; 52:1754–1756.7. Kataoka S, Liu W, Albright K, Storkson J, Pariza M. Inhibition of benzo[a]pyrene-induced mouse forestomach neoplasia and reduction of H2O2 concentration in human polymorphonuclear leucocytes by flavour components of Japanese-style fermented soy sauce. Food Chem Toxicol. 1997; 35:449–457.
Article8. Ham SS, Kim SH, Yoo SJ, Oh HT, Choi HJ, Chung MJ. Biological activities of soybean sauce (Kanjang) supplemented with deep sea water and sea tangle. Korean J Food Preserv. 2008; 15:274–279.9. Yoon KD, Kwon DJ, Hong SS, Kim SI, Chung KS. Inhibitory effect of soybean and fermented soybean products on the chemically induced mutagenesis. Korean J Appl Microbiol Bioeng. 1996; 24:525–528.10. Namiki M. Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr. 2007; 47:651–673.
Article11. Habermeyer M, Guth S, Eisenbrand G. Identification of gaps in knowledge concerning toxicology of 3-MCPD and glycidol esters. Eur J Lipid Sci Technol. 2011; 113:314–318.
Article12. Lynch BS, Bryant DW, Hook GJ, Nestmann ER, Munro IC. Carcinogenicity of monochloro-1,2-propanediol (α-chlorohydrin, 3-MCPD). Int J Toxicol. 1998; 17:47–76.
Article13. Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo. J Clin Invest. 1991; 88:1886–1893.
Article14. Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton). 2012; 17:311–321.
Article15. Salahudeen AK. Role of lipid peroxidation in H2O2-induced renal epithelial (LLC-PK1) cell injury. Am J Physiol. 1995; 268:F30–F38.
Article16. Nath KA, Salahudeen AK. Autoxidation of cysteine generates hydrogen peroxide: cytotoxicity and attenuation by pyruvate. Am J Physiol. 1993; 264:F306–F314.
Article17. Perantoni A, Berman JJ. Properties of Wilms' tumor line (TuWi) and pig kidney line (LLC-PK1) typical of normal kidney tubular epithelium. In Vitro. 1979; 15:446–454.
Article18. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010; 4:118–126.
Article19. Singh D, Kaur R, Chander V, Chopra K. Antioxidants in the prevention of renal disease. J Med Food. 2006; 9:443–450.
Article20. Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo). 1988; 36:2090–2097.
Article21. Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987; 165:215–219.
Article22. Rosen GM, Rauckman EJ. Spin trapping of superoxide and hydroxyl radicals. Methods Enzymol. 1984; 105:198–209.23. Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med. 1988; 4:155–161.
Article24. Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H2O2 solutions in the UV). Anal Biochem. 1972; 49:474–478.
Article25. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474.
Article26. Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974; 104:580–587.
Article27. Esaki H, Onozaki H, Osawa T. Antioxidative activity of fermented soybean products. In : Huang MT, Osawa T, Ho CT, Rosen RT, editors. Food Phytochemicals for Cancer Prevention I. ACS Symposium Series. Vol. 546. Washington, D.C.: American Chemical Society;1994. p. 353–360.28. Chang TS, Ding HY, Tai SS, Wu CY. Metabolism of the soy isoflavones daidzein and genistein by fungi used in the preparation of various fermented soybean foods. Biosci Biotechnol Biochem. 2007; 71:1330–1333.
Article29. Lee IH, Chou CC. Distribution profiles of isoflavone isomers in black bean kojis prepared with various filamentous fungi. J Agric Food Chem. 2006; 54:1309–1314.
Article30. Katsuzaki H, Kawakishi S, Osawa T. Sesaminol glucosides in sesame seeds. Phytochemistry. 1994; 35:773–776.
Article31. Moazzami AA, Andersson RE, Kamal-Eldin A. HPLC analysis of sesaminol glucosides in sesame seeds. J Agric Food Chem. 2006; 54:633–638.
Article32. Miyake Y, Fukumoto S, Okada M, Sakaida K, Nakamura Y, Osawa T. Antioxidative catechol lignans converted from sesamin and sesaminol triglucoside by culturing with Aspergillus. J Agric Food Chem. 2005; 53:22–27.
Article33. Song JL. Anticancer effects of fermented sesame sauce [doctor's thesis]. Busan: Pusan National University;2012.34. Liu K. Soybeans as Functional Foods and Ingredients. Champaign (IL): AOCS Press;2004.35. Dong J, Ramachandiran S, Tikoo K, Jia Z, Lau SS, Monks TJ. EGFR-independent activation of p38 MAPK and EGFR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol Renal Physiol. 2004; 287:F1049–F1058.
Article36. Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005; 33:349–357.
Article37. Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996; 16:33–50.
Article38. Braughler JM, Pregenzer JF. The 21-aminosteroid inhibitors of lipid peroxidation: reactions with lipid peroxyl and phenoxy radicals. Free Radic Biol Med. 1989; 7:125–130.
Article39. Sheridan AM, Fitzpatrick S, Wang C, Wheeler DC, Lieberthal W. Lipid peroxidation contributes to hydrogen peroxide induced cytotoxicity in renal epithelial cells. Kidney Int. 1996; 49:88–93.
Article40. Incani A, Deiana M, Corona G, Vafeiadou K, Vauzour D, Dessì MA, Spencer JP. Involvement of ERK, Akt and JNK signalling in H2O2-induced cell injury and protection by hydroxytyrosol and its metabolite homovanillic alcohol. Mol Nutr Food Res. 2010; 54:788–796.
Article41. Hou RC, Huang HM, Tzen JT, Jeng KC. Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J Neurosci Res. 2003; 74:123–133.
Article42. Kang MH, Naito M, Tsujihara N, Osawa T. Sesamolin inhibits lipid peroxidation in rat liver and kidney. J Nutr. 1998; 128:1018–1022.
Article43. Sirato-Yasumoto S, Katsuta M, Okuyama Y, Takahashi Y, Ide T. Effect of sesame seeds rich in sesamin and sesamolin on fatty acid oxidation in rat liver. J Agric Food Chem. 2001; 49:2647–2651.
Article44. Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol. 2000; 164:224–229.
Article45. Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004; 10:RA141–RA147.46. Comporti M. Glutathione depleting agents and lipid peroxidation. Chem Phys Lipids. 1987; 45:143–169.
Article47. Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012; 132:931–935.
Article48. Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004; 6:289–300.
Article49. Yu MU, Yoo JM, Lee YS, Lee YM, Hong JT, Oh KW, Song S, Yun YP, Yoo HS, Oh S. Altered de novo sphingolipid biosynthesis is involved in the serum deprivation-induced cell death in LLC-PK1 cells. J Toxicol Environ Health A. 2004; 67:2085–2094.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidative effects of Kimchi under different fermentation stage on radical-induced oxidative stress
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- The Effects of Oxalate on the DNA Synthesis in LLC-PK1 Cells
- Protective effect of fucoidan against tacrolimus-induced nephrotoxicity in LLC-PK1 cells
- Role of Ascorbic Acid Against the Oxidative Stress in Trabecular Meshwork Cells