Korean J Hematol.
2007 Sep;42(3):224-232. 10.5045/kjh.2007.42.3.224.
Aging Effects on Dendritic Cells after Total Body Irradiation in Mice
- Affiliations
-
- 1Department of Hematology-Oncology, Ajou University School of Medicine, Suwon, Korea. jspark65@ajou.ac.kr
- KMID: 2305197
- DOI: http://doi.org/10.5045/kjh.2007.42.3.224
Abstract
-
BACKGROUND: It is still obscure how dendritic cells (DCs) can orchestrate whole immune reactions according to the host age. We studied changes of murine splenic DCs after total body irradiation (TBI), with regards to age.
METHODS
Young (8~14 wk) and old (12~16 mo) C57Bl/6 mice were irradiated with a dose of 1,100 cGy and were assessed 6 h later for phenotypic and functional changes of the DCs. The mean fluorescence intensities and cytokine producing cell proportions were analyzed with the student's t-test.
RESULTS
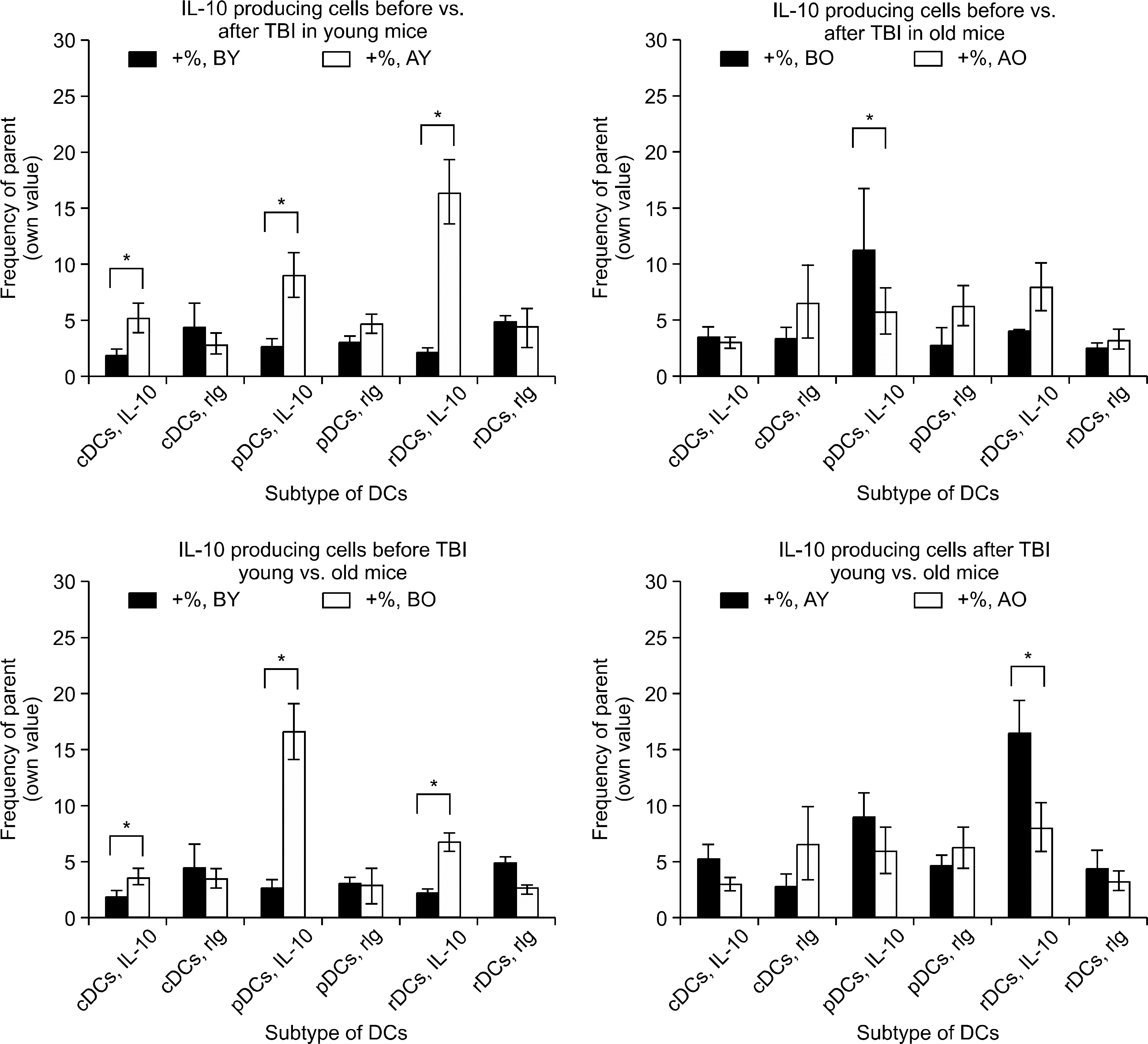
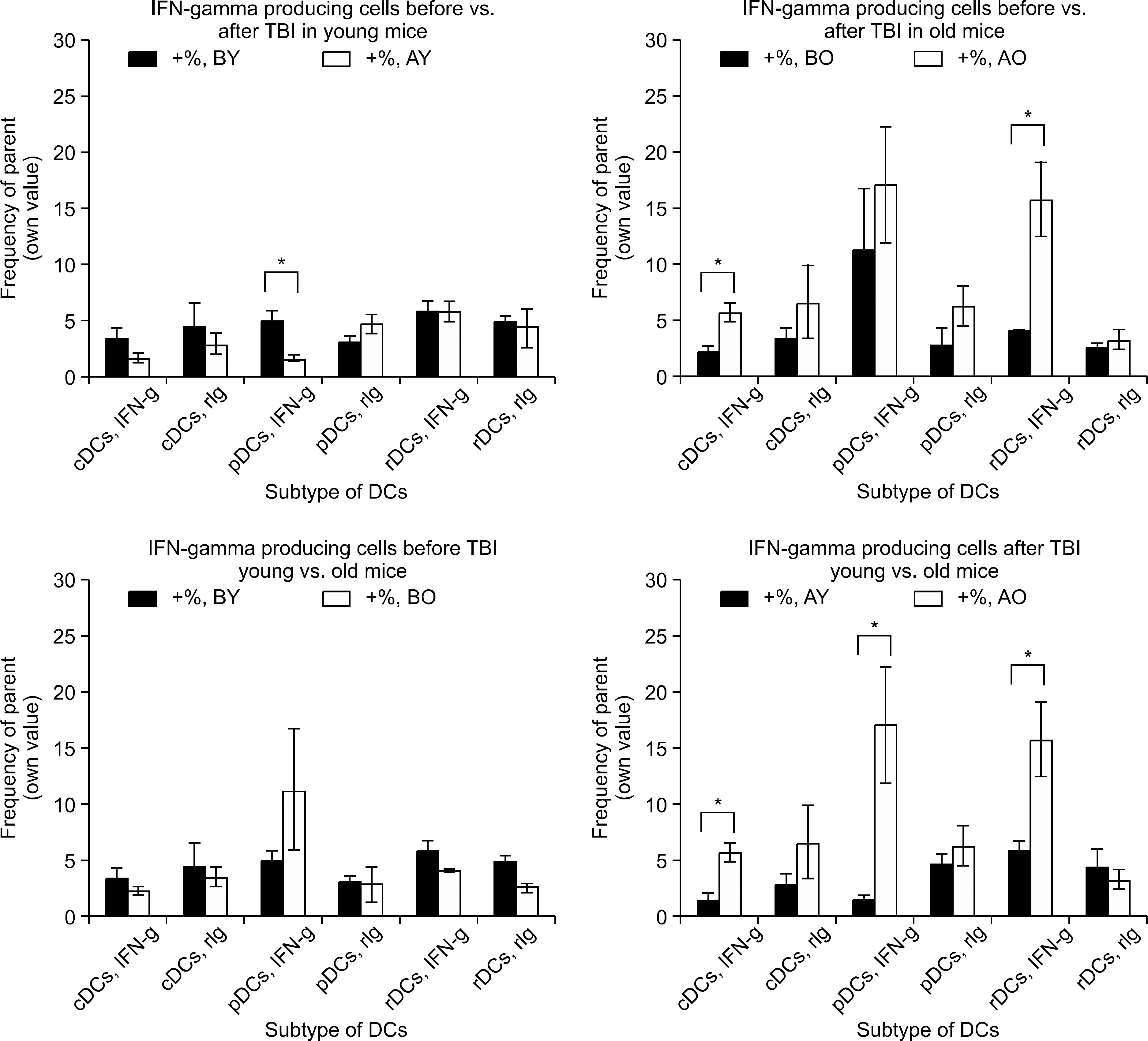
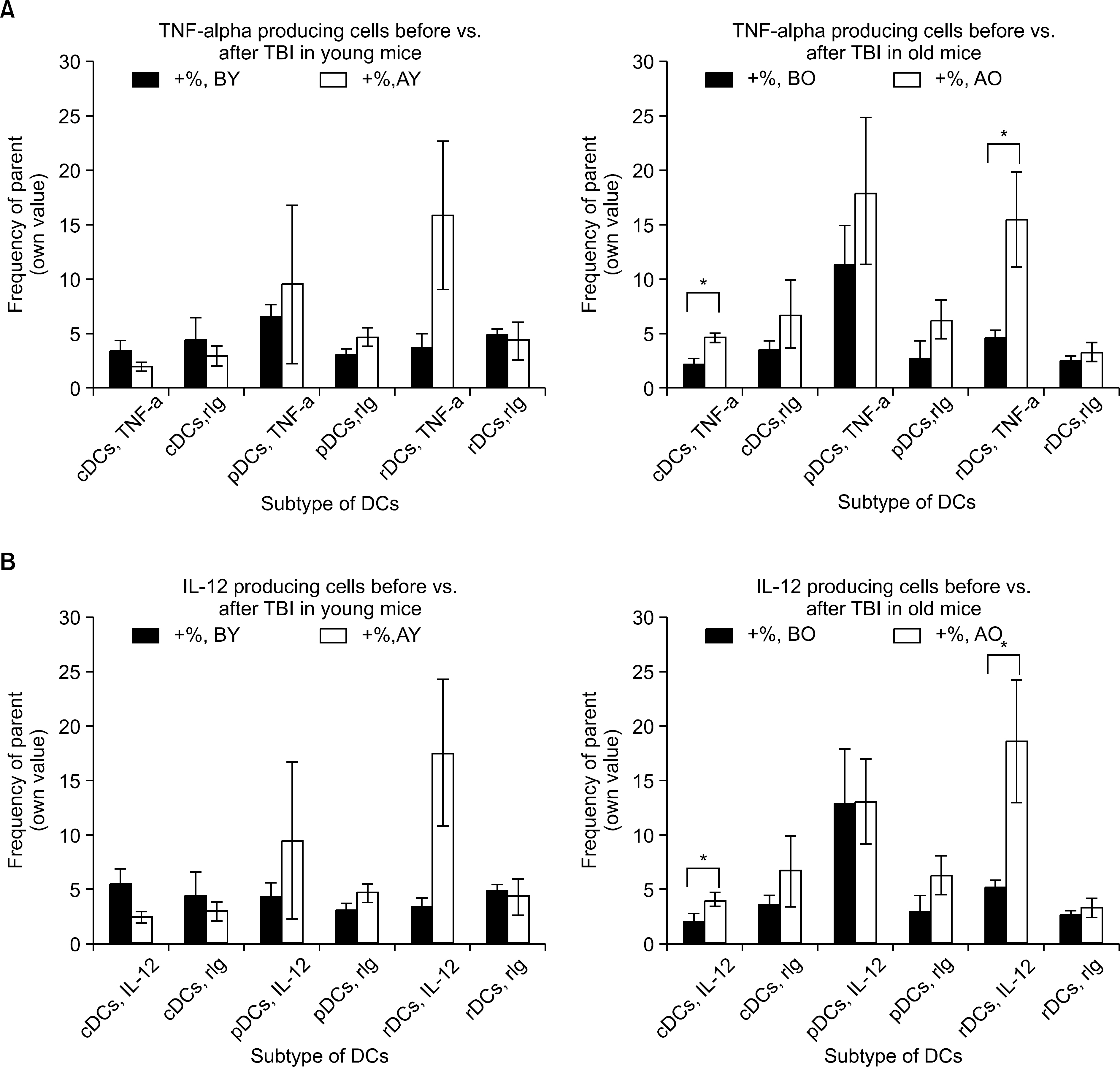
Interleukin-12 (IL-12), interferon (IFN gamma) and tumor necrosis factor (TNF alpha) producing classical DCs (cDCs) were more numerous in the young untreated mice than in the old mice. However, the number of these cells decreased in the young mice and increased in the old mice after TBI. IL-12, IFN gamma and TNF alpha producing plasmacytoid DCs (pDCs) were more frequent in the old mice than in the young mice before TBI both mice showed an increased frequency of cells producing these cytokines after TBI. Overall, the highest numbers of cDCs and pDCs producing IL-12, IFN gamma and TNF alpha were present in the old mice after TBI. In both the cDC and pDC populations, the old mice had a higher frequency of IL-10+ cells prior to TBI. After irradiation, the young mice had a higher frequency of IL-10+ cells.
CONCLUSION
With TBI, the DCs showed dramatic differences between young and old mice. Young mice turned to an immuno-suppressive response whereas the old mice changed to an immuno-stimulation of DCs after TBI. From these dramatic aging effects, we hope to explain the different frequencies and severities of acute GvHD after allogeneic hematopoietic stem cell transplantation according to host age.
Keyword
MeSH Terms
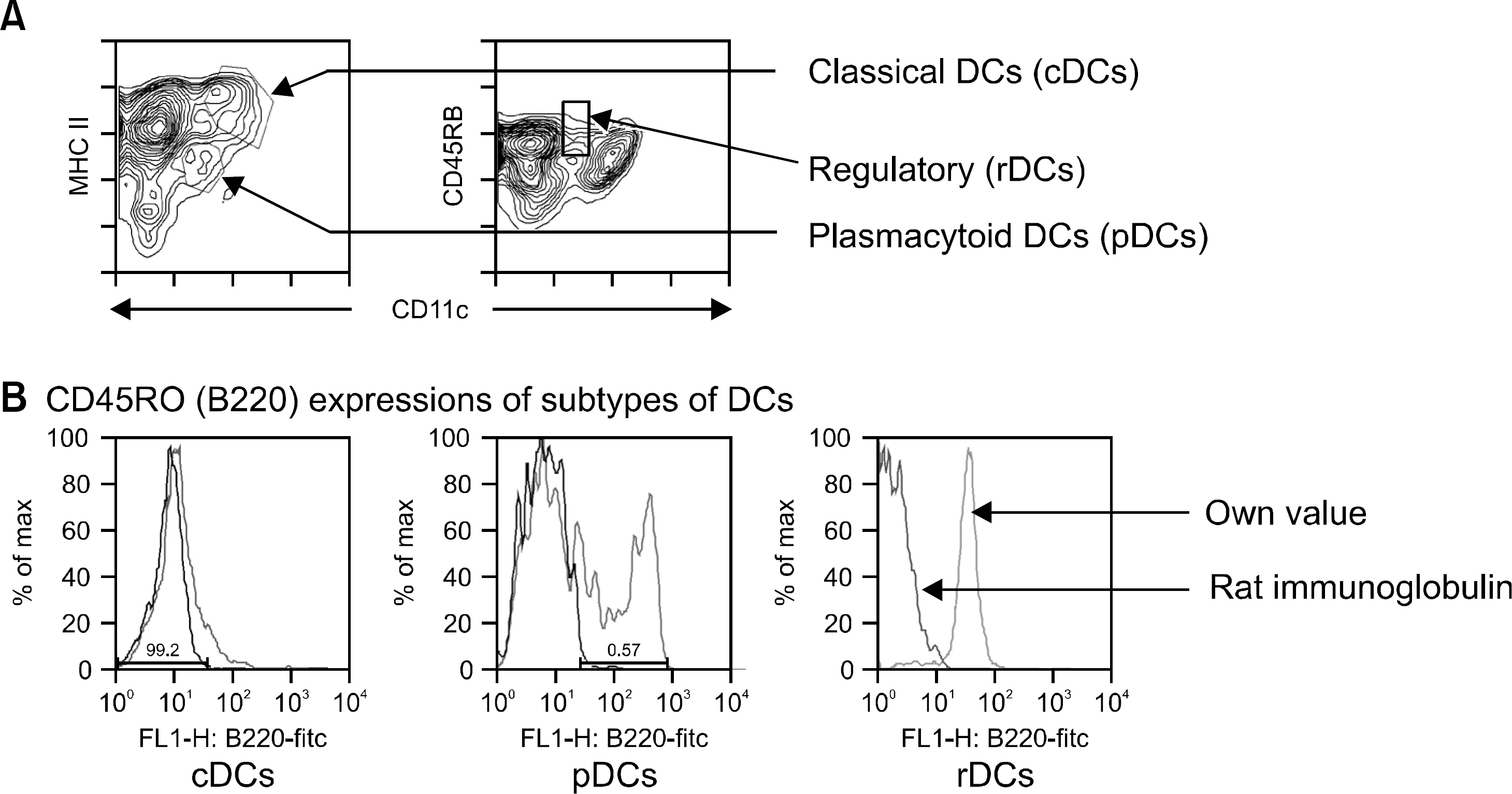
Figure
Reference
-
1). Maraninchi D., Gluckman E., Blaise D, et al. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukae-mias. Lancet. 1987. 2:175–8.
Article2). Marmont AM., Horowitz MM., Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991. 78:2120–30.
Article3). Sullivan K., Storb R., Buckner CD, et al. Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N Engl J Med. 1989. 320:828–34.
Article4). Bryson JS., Jennings CD., Caywood BE., Dix AR., Lowery DM., Kaplan AM. Enhanced graft-versus-host disease in older recipient mice following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997. 19:721–8.
Article5). Storb R. Graft rejection and graft-versus-host disease in marrow transplantation. Transplant Proc. 1989. 21:2915–8.6). Shlomchik WD., Couzens MS., Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999. 285:412–5.
Article7). Steinman RM., Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973. 137:1142–62.8). Steinman RM., Lusting DS., Cohn ZA. Identification of a novel cell type in peripheral lympoid organs of mice. III. Functional properties in vivo. J Exp Med. 1974. 139:1431–45.9). Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991. 9:271–96.
Article10). Wakkach A., Fournier N., Brun V., Breittmayer JP., Cottrez F., Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003. 18:605–17.
Article11). Sato K., Yamashita N., Baba M., Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood. 2003. 101:3581–9.
Article12). Sato K., Yamashita N., Yamashita N., Baba M., Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003. 18:367–79.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Response to Co-60 Total Body Irradiation (TBI) With 600 cgy at 3 Different Does Rates in the Mice
- Effects of X-irradiation on the levels of serum cholesterol in mouse
- Effects of same TDF Factors on Body Weight of Mice and Peripheral Blood Picture
- Defects in the differentiation and function of bone marrow-derived dendritic cells in non-obese diabetic mice
- Aspectual Comparison of the Skin Changes in Hairless Mice According to the Aging Type