Korean J Physiol Pharmacol.
2015 Mar;19(2):183-189. 10.4196/kjpp.2015.19.2.183.
Foeniculum vulgare Mill. Protects against Lipopolysaccharide-induced Acute Lung Injury in Mice through ERK-dependent NF-kB Activation
- Affiliations
-
- 1Department of Basic Nursing Science, School of Nursing, Korea University, Seoul 136-701, Korea. ghseol@korea.ac.kr
- KMID: 2285571
- DOI: http://doi.org/10.4196/kjpp.2015.19.2.183
Abstract
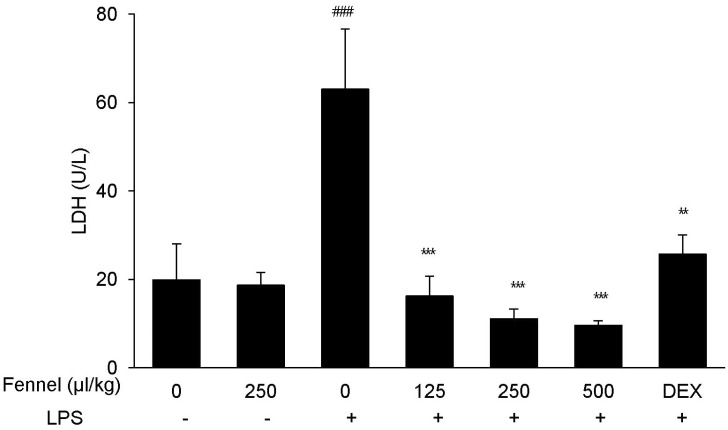
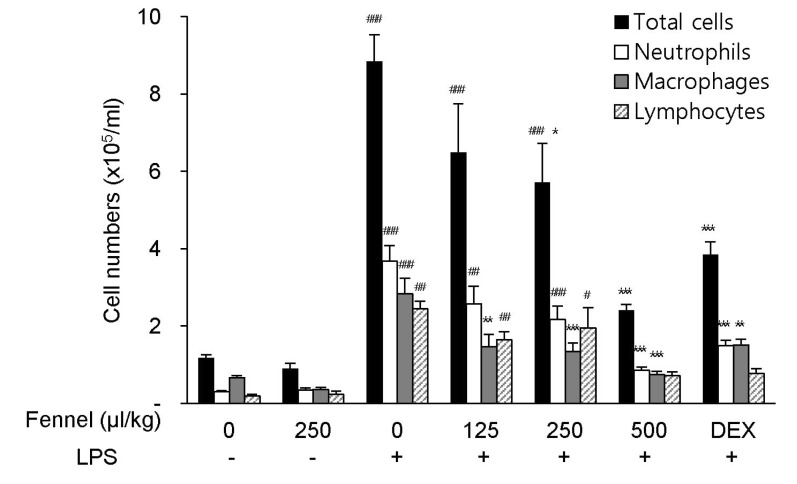
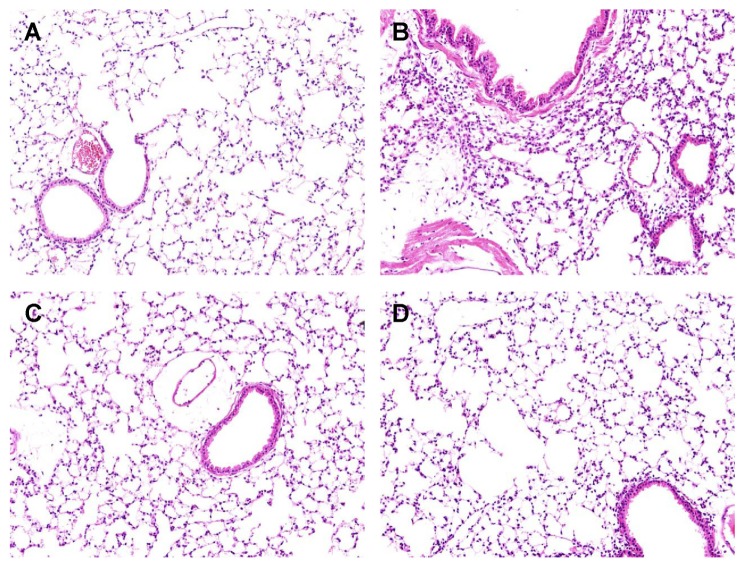
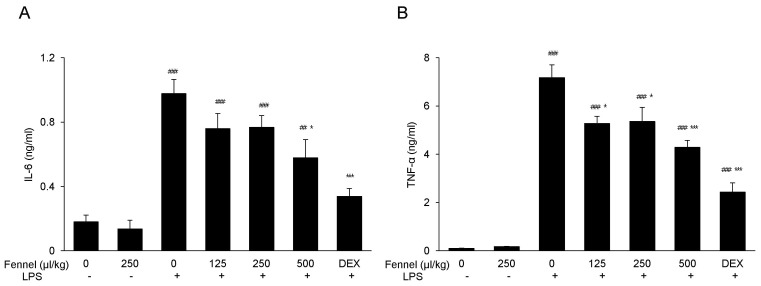
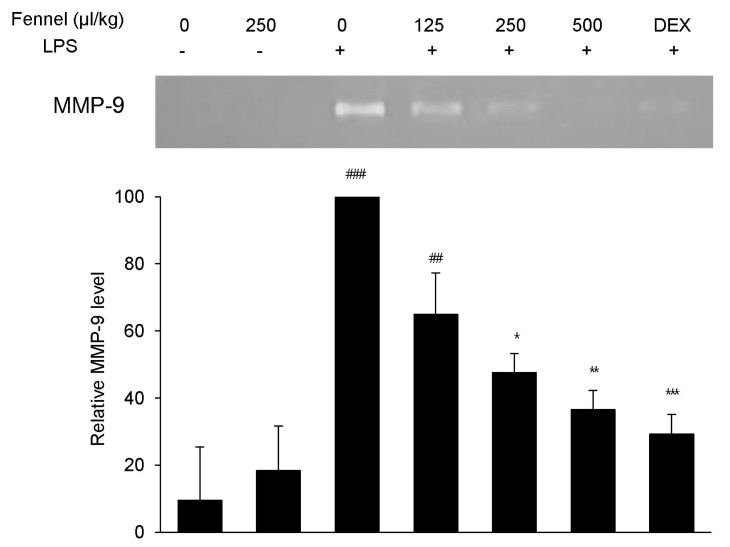
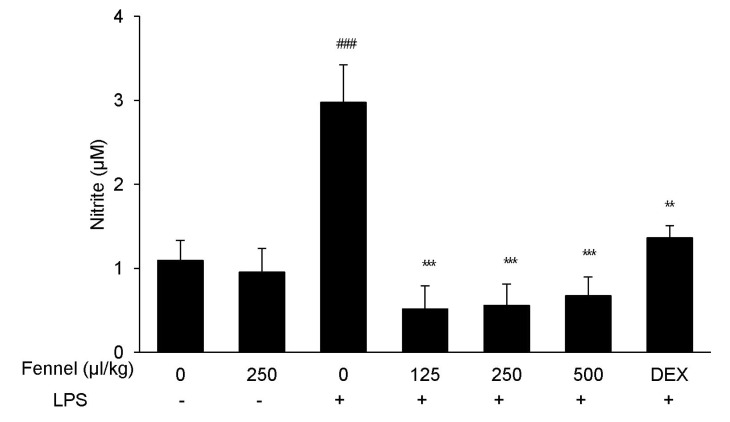
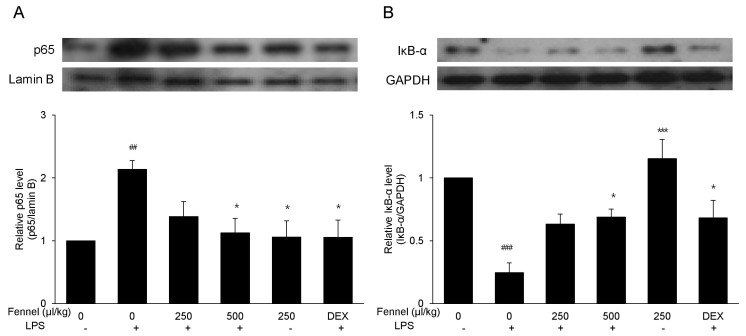
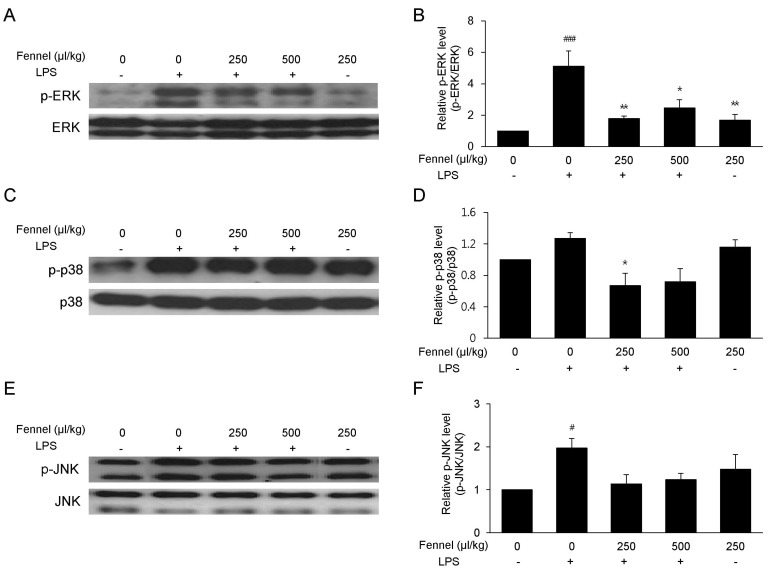
- Foeniculum vulgare Mill. (fennel) is used to flavor food, in cosmetics, as an antioxidant, and to treat microbial, diabetic and common inflammation. No study to date, however, has assessed the anti-inflammatory effects of fennel in experimental models of inflammation. The aims of this study were to investigate the anti-inflammatory effects of fennel in model of lipopolysaccharide (LPS)-induced acute lung injury. Mice were randomly assigned to seven groups (n=7~10). In five groups, the mice were intraperitoneally injected with 1% Tween 80-saline (vehicle), fennel (125, 250, 500micro l/kg), or dexamethasone (1 mg/kg), followed 1 h later by intratracheal instillation of LPS (1.5 mg/kg). In two groups, the mice were intraperitoneally injected with vehicle or fennel (250microl/kg), followed 1 h later by intratracheal instillation of sterile saline. Mice were sacrificed 4 h later, and bronchoalveolar lavage fluid (BALF) and lung tissues were obtained. Fennel significantly and dose-dependently reduced LDH activity and immune cell numbers in LPS treated mice. In addition fennel effectively suppressed the LPS-induced increases in the production of the inflammatory cytokines interleukin-6 and tumor necrosis factor-alpha, with 500microl/kg fennel showing maximal reduction. Fennel also significantly and dose-dependently reduced the activity of the proinflammatory mediator matrix metalloproteinase 9 and the immune modulator nitric oxide (NO). Assessments of the involvement of the MAPK signaling pathway showed that fennel significantly decreased the LPS-induced phosphorylation of ERK. Fennel effectively blocked the inflammatory processes induced by LPS, by regulating pro-inflammatory cytokine production, transcription factors, and NO.
Keyword
MeSH Terms
-
Acute Lung Injury*
Animals
Bronchoalveolar Lavage Fluid
Cell Count
Cytokines
Dexamethasone
Foeniculum*
Inflammation
Interleukin-6
Lung
Matrix Metalloproteinase 9
Mice*
Models, Theoretical
NF-kappa B*
Nitric Oxide
Phosphorylation
Transcription Factors
Tumor Necrosis Factor-alpha
Cytokines
Dexamethasone
Interleukin-6
Matrix Metalloproteinase 9
NF-kappa B
Nitric Oxide
Transcription Factors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Herold S, Gabrielli NM, Vadász I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2013; 305:L665–L681. PMID: 24039257.
Article2. Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J. 2011; 87:612–622. PMID: 21642654.
Article3. Kabir K, Gelinas JP, Chen M, Chen D, Zhang D, Luo X, Yang JH, Carter D, Rabinovici R. Characterization of a murine model of endotoxin-induced acute lung injury. Shock. 2002; 17:300–303. PMID: 11954830.
Article4. Mizumura K, Gon Y, Kumasawa F, Onose A, Maruoka S, Matsumoto K, Hayashi S, Kobayashi T, Hashimoto S. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates lipopolysaccharide-induced tissue factor expression in pulmonary microvasculature. Int Immunopharmacol. 2010; 10:1062–1067. PMID: 20601186.
Article5. Goswami N, Chatterjee S. Assessment of free radical scavenging potential and oxidative DNA damage preventive activity of Trachyspermum ammi L. (carom) and Foeniculum vulgare Mill. (fennel) seed extracts. Biomed Res Int. 2014; 2014:582767. PMID: 25143939.6. Choi EM, Hwang JK. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia. 2004; 75:557–565. PMID: 15351109.
Article7. Mohamad RH, El-Bastawesy AM, Abdel-Monem MG, Noor AM, Al-Mehdar HA, Sharawy SM, El-Merzabani MM. Antioxidant and anticarcinogenic effects of methanolic extract and volatile oil of fennel seeds (Foeniculum vulgare). J Med Food. 2011; 14:986–1001. PMID: 21812646.
Article8. Ritter AM, Domiciano TP, Verri WA Jr, Zarpelon AC, da Silva LG, Barbosa CP, Natali MR, Cuman RK, Bersani-Amado CA. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology. 2013; 21:187–197. PMID: 23054333.
Article9. Kang P, Kim KY, Lee HS, Min SS, Seol GH. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013; 93:955–961. PMID: 24404587.
Article10. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138. PMID: 7181105.
Article11. Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997; 2:d12–d26. PMID: 9159205.
Article12. Gao MY, Chen L, Yang L, Yu X, Kou JP, Yu BY. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol Rep. 2014; 66:480–484. PMID: 24905527.
Article13. Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001; 167:5067–5076. PMID: 11673516.
Article14. Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004; 382:393–409. PMID: 15214841.15. Cho HI, Kim KM, Kwak JH, Lee SK, Lee SM. Protective mechanism of anethole on hepatic ischemia/reperfusion injury in mice. J Nat Prod. 2013; 76:1717–1723. PMID: 23962021.
Article16. Zhang T, Feng Q. Nitric oxide and calcium signaling regulate myocardial tumor necrosis factor-α expression and cardiac function in sepsis. Can J Physiol Pharmacol. 2010; 88:92–104. PMID: 20237583.17. Geoghegan-Morphet N, Burger D, Lu X, Sathish V, Peng T, Sims SM, Feng Q. Role of neuronal nitric oxide synthase in lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal mouse cardiomyocytes. Cardiovasc Res. 2007; 75:408–416. PMID: 17466955.18. Qiu J, Li H, Su H, Dong J, Luo M, Wang J, Leng B, Deng Y, Liu J, Deng X. Chemical composition of fennel essential oil and its impact on Staphylococcus aureus exotoxin production. World J Microbiol Biotechnol. 2012; 28:1399–1405. PMID: 22805920.
Article19. Boskabady MH, Khatami A, Nazari A. Possible mechanism(s) for relaxant effects of Foeniculum vulgare on guinea pig tracheal chains. Pharmazie. 2004; 59:561–564. PMID: 15296096.20. Soares PM, Lima RF, de Freitas Pires A, Souza EP, Assreuy AM, Criddle DN. Effects of anethole and structural analogues on the contractility of rat isolated aorta: Involvement of voltage-dependent Ca2+-channels. Life Sci. 2007; 81:1085–1093. PMID: 17869309.21. Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014; 26:237–245. PMID: 24647229.
Article22. Moncada S, Higgs EA. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995; 9:1319–1330. PMID: 7557022.
Article23. Rehman MU, Tahir M, Khan AQ, Khan R, Oday-O-Hamiza , Lateef A, Hassan SK, Rashid S, Ali N, Zeeshan M, Sultana S. D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Exp Biol Med (Maywood). 2014; 239:465–476. PMID: 24586096.
Article24. d'Alessio PA, Ostan R, Bisson JF, Schulzke JD, Ursini MV, Béné MC. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013; 92:1151–1156. PMID: 23665426.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Signaling Pathway of the Bacterial Lipopolysaccharide-induced Generation of Nitric Oxide in Rat Primary Astorcytes
- Aromadendrin Inhibits Lipopolysaccharide-Induced Inflammation in BEAS-2B Cells and Lungs of Mice
- Protective Effect of Nitric Oxide Against Lipopolysaccharide-induced Cytotoxicity in C6-glial Cell
- Toll-like receptor 9 dependent activation of MAPK and NF-kB is required for the CpG ODN-induced matrix metalloproteinase-9 expression
- p38 MAPK and NF-kappaB are required for LPS-induced RANTES production in immortalized murine microglia (BV-2)