Korean J Physiol Pharmacol.
2011 Dec;15(6):397-403. 10.4196/kjpp.2011.15.6.397.
Cigarette Smoke Extract-induced Reduction in Migration and Contraction in Normal Human Bronchial Smooth Muscle Cells
- Affiliations
-
- 1Department of Physiology, Gyeongsang National University School of Medicine, Jinju 660-751, Korea. dawon@gnu.ac.kr, jheehan@gnu.ac.kr
- 2Department of Rehabilitation Medicine, Gyeongsang National University School of Medicine, Jinju 660-751, Korea.
- 3Department of Respiratory Medicine, Gyeongsang National University School of Medicine, Jinju 660-751, Korea.
- 4Department of Biochemistry and Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-751, Korea.
- KMID: 2285427
- DOI: http://doi.org/10.4196/kjpp.2011.15.6.397
Abstract
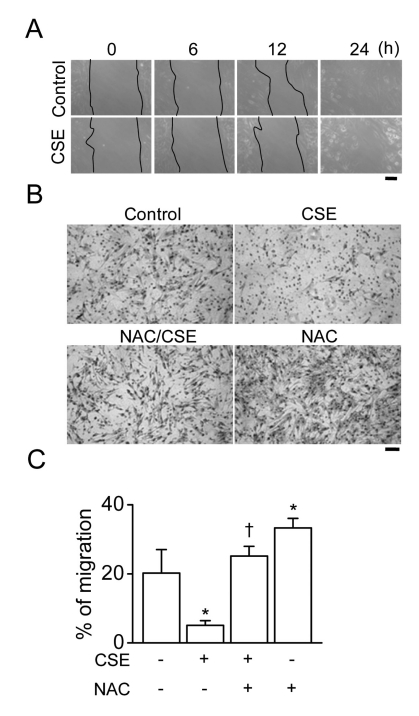
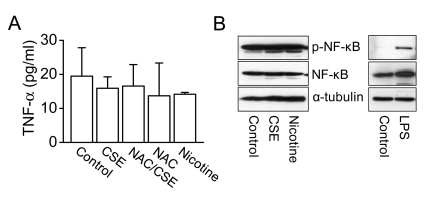
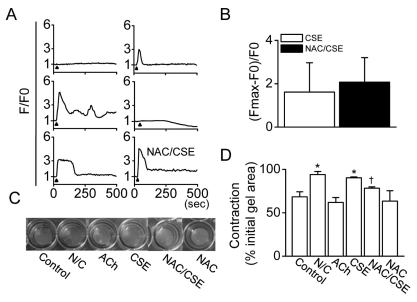
- The proliferation, migration, cytokine release, and contraction of airway smooth muscle cells are key events in the airway remodeling process that occur in lung disease such as asthma, chronic obstruction pulmonary disease, and cancer. These events can be modulated by a number of factors, including cigarette smoke extract (CSE). CSE-induced alterations in the viability, migration, and contractile abilities of normal human airway cells remain unclear. This study investigated the effect of CSE on cell viability, migration, tumor necrosis factor (TNF)-alpha secretion, and contraction in normal human bronchial smooth muscle cells (HBSMCs). Treatment of HBSMCs with 10% CSE induced cell death, and the death was accompanied by the generation of reactive oxygen species (ROS). CSE-induced cell death was reduced by N-acetyl-l-cysteine (NAC), an ROS scavenger. In addition, CSE reduced the migration ability of HBSMCs by 75%. The combination of NAC with CSE blocked the CSE-induced reduction of cell migration. However, CSE had no effect on TNF-alpha secretion and NF-kappaB activation. CSE induced an increase in intracellular Ca2+ concentration in 64% of HBSMCs. CSE reduced the contractile ability of HBSMCs, and the ability was enhanced by NAC treatment. These results demonstrate that CSE treatment induces cell death and reduces migration and contraction by increasing ROS generation in normal HBSMCs. These results suggest that CSE may induce airway change through cell death and reduction in migration and contraction of normal HBSMCs.
MeSH Terms
-
Acetylcysteine
Airway Remodeling
Asthma
Bronchioles
Cell Death
Cell Movement
Cell Survival
Contracts
Emigration and Immigration
Humans
Lung Diseases
Muscle, Smooth
Myocytes, Smooth Muscle
NF-kappa B
Reactive Oxygen Species
Smoke
Tobacco Products
Tumor Necrosis Factor-alpha
Acetylcysteine
NF-kappa B
Reactive Oxygen Species
Smoke
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Regulation of Ca2+ Signaling in Pulmonary Hypertension
Amy L. Firth, Jun Yeon Won, Won Sun Park
Korean J Physiol Pharmacol. 2013;17(1):1-8. doi: 10.4196/kjpp.2013.17.1.1.
Reference
-
1. Banerjee S, Chattopadhyay R, Ghosh A, Koley H, Panda K, Roy S, Chattopadhyay D, Chatterjee IB. Cellular and molecular mechanisms of cigarette smoke-induced lung damage and prevention by vitamin C. J Inflamm (Lond). 2008; 5:21. PMID: 19014449.
Article2. Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993; 686:12–27. PMID: 8512242.
Article3. Tang ML, Wilson JW, Stewart AG, Royce SG. Airway remodelling in asthma: current understanding and implications for future therapies. Pharmacol Ther. 2006; 112:474–488. PMID: 16759709.
Article4. Xu W, Hong W, Shao Y, Ning Y, Cai Z, Li Q. Nogo-B regulates migration and contraction of airway smooth muscle cells by decreasing ARPC 2/3 and increasing MYL-9 expression. Respir Res. 2011; 12:14. PMID: 21251247.
Article5. An SS, Fredberg JJ. Biophysical basis for airway hyperresponsiveness. Can J Physiol Pharmacol. 2007; 85:700–714. PMID: 17823634.6. Sumi Y, Hamid Q. Airway remodeling in asthma. Allergol Int. 2007; 56:341–348. PMID: 17965577.
Article7. Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007; 19:676–680. PMID: 17720466.
Article8. Jeong SH, Park JH, Kim JN, Park YH, Shin SY, Lee YH, Kye YC, Son SW. Up-regulation of TNF-alpha secretion by cigarette smoke is mediated by Egr-1 in HaCaT human keratinocytes. Exp Dermatol. 2010; 19:e206–e212. PMID: 20653771.
Article9. Preciado D, Kuo E, Ashktorab S, Manes P, Rose M. Cigarette smoke activates NFκB-mediated Tnf-α release from mouse middle ear cells. Laryngoscope. 2010; 120:2508–2515. PMID: 21108432.
Article10. Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004; 56:515–548. PMID: 15602009.
Article11. Jeong YY, Park HJ, Cho YW, Kim EJ, Kim GT, Mun YJ, Lee JD, Shin JH, Sung NJ, Kang D, Han J. Aged red garlic extract reduces cigarette smoke extract-induced cell death in human bronchial smooth muscle cells by increasing intracellular glutathione levels. Phytother Res. 2011.
Article12. Li QF, Tang DD. Role of p47(phox) in regulating Cdc42GAP, vimentin, and contraction in smooth muscle cells. Am J Physiol Cell Physiol. 2009; 297:C1424–C1433. PMID: 19812368.13. van der Vaart H, Postma DS, Timens W, ten Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004; 59:713–721. PMID: 15282395.
Article14. Marthan R, Roux E, Savineau JP. Human bronchial smooth muscle responsiveness after in vitro exposure to oxidizing pollutants. Cell Biol Toxicol. 1996; 12:245–249. PMID: 9034616.
Article15. Ben-Jebria A, Marthan R, Rossetti M, Savineau JP, Ultman JS. Effect of in vitro exposure to acrolein on carbachol responses in rat trachealis muscle. Respir Physiol. 1993; 93:111–123. PMID: 8367612.16. Xu LJ, Dandurand RJ, Lei M, Eidelman DH. Airway hyperresponsiveness in cigarette smoke-exposed rats. Lung. 1993; 171:95–107. PMID: 8426466.
Article17. Wu ZX, Lee LY. Airway hyperresponsiveness induced by chronic exposure to cigarette smoke in guinea pigs: role of tachykinins. J Appl Physiol. 1999; 87:1621–1628. PMID: 10562600.18. Barrett EG, Wilder JA, March TH, Espindola T, Bice DE. Cigarette smoke-induced airway hyperresponsiveness is not dependent on elevated immunoglobulin and eosinophilic inflammation in a mouse model of allergic airway disease. Am J Respir Crit Care Med. 2002; 165:1410–1418. PMID: 12016105.
Article19. Streck E, Jörres RA, Huber RM, Bergner A. Effects of cigarette smoke extract and nicotine on bronchial tone and acetylcholine-induced airway contraction in mouse lung slices. J Investig Allergol Clin Immunol. 2010; 20:324–330.20. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997; 49:157–230. PMID: 9228665.21. Baglole CJ, Sime PJ, Phipps RP. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. Am J Physiol Lung Cell Mol Physiol. 2008; 295:L624–L636. PMID: 18689604.
Article22. Nana-Sinkam SP, Lee JD, Sotto-Santiago S, Stearman RS, Keith RL, Choudhury Q, Cool C, Parr J, Moore MD, Bull TM, Voelkel NF, Geraci MW. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med. 2007; 175:676–685. PMID: 17255567.
Article23. Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol. 2010; 185:3035–3040. PMID: 20660708.
Article24. Lau WK, Chan SC, Law AC, Ip MS, Mak JC. The role of MAPK and Nrf2 pathways in ketanserin-elicited attenuation of cigarette smoke-induced IL-8 production in human bronchial epithelial cells. Toxicol Sci. 2011.
Article25. Lee SA, Kim HJ, Chang KC, Baek JC, Park JK, Shin JK, Choi WJ, Lee JH, Paik WY. DHA and EPA down-regulate COX-2 expression through suppression of NF-kappaB activity in LPS-treated human umbilical vein endothelial cells. Korean J Physiol Pharmacol. 2009; 13:301–307. PMID: 19885014.26. Lee DH, Choi HC, Lee KY, Kang YJ. Aprotinin inhibits vascular smooth muscle cell inflammation and proliferation via induction of HO-1. Korean J Physiol Pharmacol. 2009; 13:123–129. PMID: 19885007.
Article27. Pera T, Gosens R, Lesterhuis AH, Sami R, Toorn M, Zaagsma J, Meurs H. Cigarette smoke and lipopolysaccharide induce a proliferative airway smooth muscle phenotype. Respir Res. 2010; 11:48. PMID: 20429916.
Article28. Xu CB, Lei Y, Chen Q, Pehrson C, Larsson L, Edvinsson L. Cigarette smoke extracts promote vascular smooth muscle cell proliferation and enhances contractile responses in the vasculature and airway. Basic Clin Pharmacol Toxicol. 2010; 107:940–948. PMID: 20618305.
Article29. Hu W, Xie J, Zhao J, Xu Y, Yang S, Ni W. Involvement of Bcl-2 family in apoptosis and signal pathways induced by cigarette smoke extract in the human airway smooth muscle cells. DNA Cell Biol. 2009; 28:13–22. PMID: 19090673.
Article30. Oltmanns U, Chung KF, Walters M, John M, Mitchell JA. Cigarette smoke induces IL-8, but inhibits eotaxin and RANTES release from airway smooth muscle. Respir Res. 2005; 6:74. PMID: 16029496.
Article31. Sandborn WJ. Nicotine therapy for ulcerative colitis: a review of rationale, mechanisms, pharmacology, and clinical results. Am J Gastroenterol. 1999; 94:1161–1171. PMID: 10235187.
Article32. Goerig M, Ullrich V, Schettler G, Foltis C, Habenicht A. A new role for nicotine: selective inhibition of thromboxane formation by direct interaction with thromboxane synthase in human promyelocytic leukaemia cells differentiating into macrophages. Clin Investig. 1992; 70:239–243.
Article33. Tsoyi K, Jang HJ, Kim JW, Chang HK, Lee YS, Pae HO, Kim HJ, Seo HG, Lee JH, Chung HT, Chang KC. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011; 14:2057–2070. PMID: 21083424.
Article34. Joe Y, Kim HJ, Kim S, Chung J, Ko MS, Lee WH, Chang KC, Park JW, Chung HT. Tristetraprolin mediates anti-inflammatory effects of nicotine in lipopolysaccharide-stimulated macrophages. J Biol Chem. 2011; 286:24735–24742. PMID: 21606497.
Article35. Yoshiyama S, Horinouchi T, Miwa S, Wang HH, Kohama K, Nakamura A. Effect of cigarette smoke components on vascular smooth muscle cell migration toward platelet-derived growth factor BB. J Pharmacol Sci. 2011; 115:532–535. PMID: 21422730.
Article36. Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004; 18:1897–1899. PMID: 15456740.37. Vayssier M, Favatier F, Pinot F, Bachelet M, Polla BS. Tobacco smoke induces coordinate activation of HSF and inhibition of NFkappaB in human monocytes: effects on TNFalpha release. Biochem Biophys Res Commun. 1998; 252:249–256. PMID: 9813178.38. Cheng SE, Luo SF, Jou MJ, Lin CC, Kou YR, Lee IT, Hsieh HL, Yang CM. Cigarette smoke extract induces cytosolic phospholipase A2 expression via NADPH oxidase, MAPKs, AP-1, and NF-kappaB in human tracheal smooth muscle cells. Free Radic Biol Med. 2009; 46:948–960. PMID: 19280714.39. Yang CM, Lee IT, Lin CC, Yang YL, Luo SF, Kou YR, Hsiao LD. Cigarette smoke extract induces COX-2 expression via a PKCalpha/c-Src/EGFR, PDGFR/PI3K/Akt/NF-kappaB pathway and p300 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L892–L902. PMID: 19717552.40. Chiba Y, Murata M, Ushikubo H, Yoshikawa Y, Saitoh A, Sakai H, Kamei J, Misawa M. Effect of cigarette smoke exposure in vivo on bronchial smooth muscle contractility in vitro in rats. Am J Respir Cell Mol Biol. 2005; 33:574–581. PMID: 16166743.41. Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008; 118:2574–2582. PMID: 18568077.42. Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006; 133:4355–4365. PMID: 17038520.43. Cho SK, Yoon SY, Hur CG, Yang HY, Choe C, Kim EJ, Joo JS, Kang KR, Park JY, Hong SG, Han J, Kang D. Acetylcholine rescues two-cell block through activation of IP3 receptors and Ca2+/calmodulin-dependent kinase II in an ICR mouse strain. Pflugers Arch. 2009; 458:1125–1136. PMID: 19484474.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Halothane and Enflurane on the Contraction of Bronchial Smooth Muscle Induced by Histamine in Rabbits
- Effect of Panax Ginseng Alcohol Extract on Cardiovascular System
- Effects of Antioxidant on Oxidative Stress and Autophagy in Bronchial Epithelial Cells Exposed to Particulate Matter and Cigarette Smoke Extract
- Mechanism of an increase in concentration of intracellular calcium by carbachol in human gastric smooth muscle cell
- The Effects of Heparin and Protamine on Contraction of Tracheal Smooth Muscle Induced by Carbachol in the Guinea Pig