Chonnam Med J.
2012 Apr;48(1):7-14. 10.4068/cmj.2012.48.1.7.
The Expression of Toll-Like Receptors (TLRs) in Cultured Human Skin Fibroblast is Modulated by Histamine
- Affiliations
-
- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea. seongkim@jnu.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2274875
- DOI: http://doi.org/10.4068/cmj.2012.48.1.7
Abstract
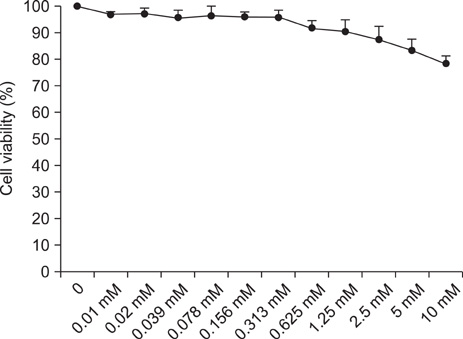
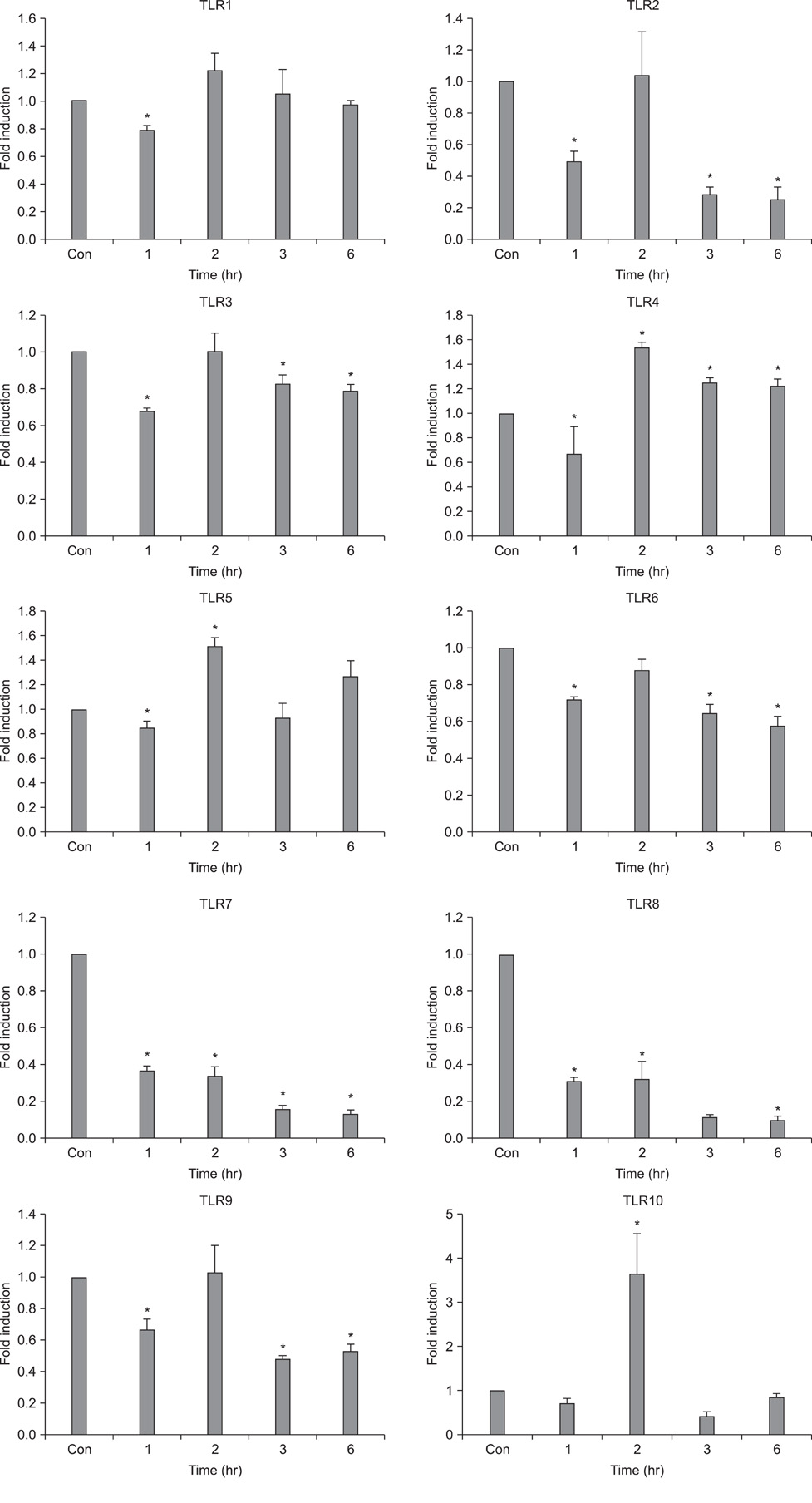
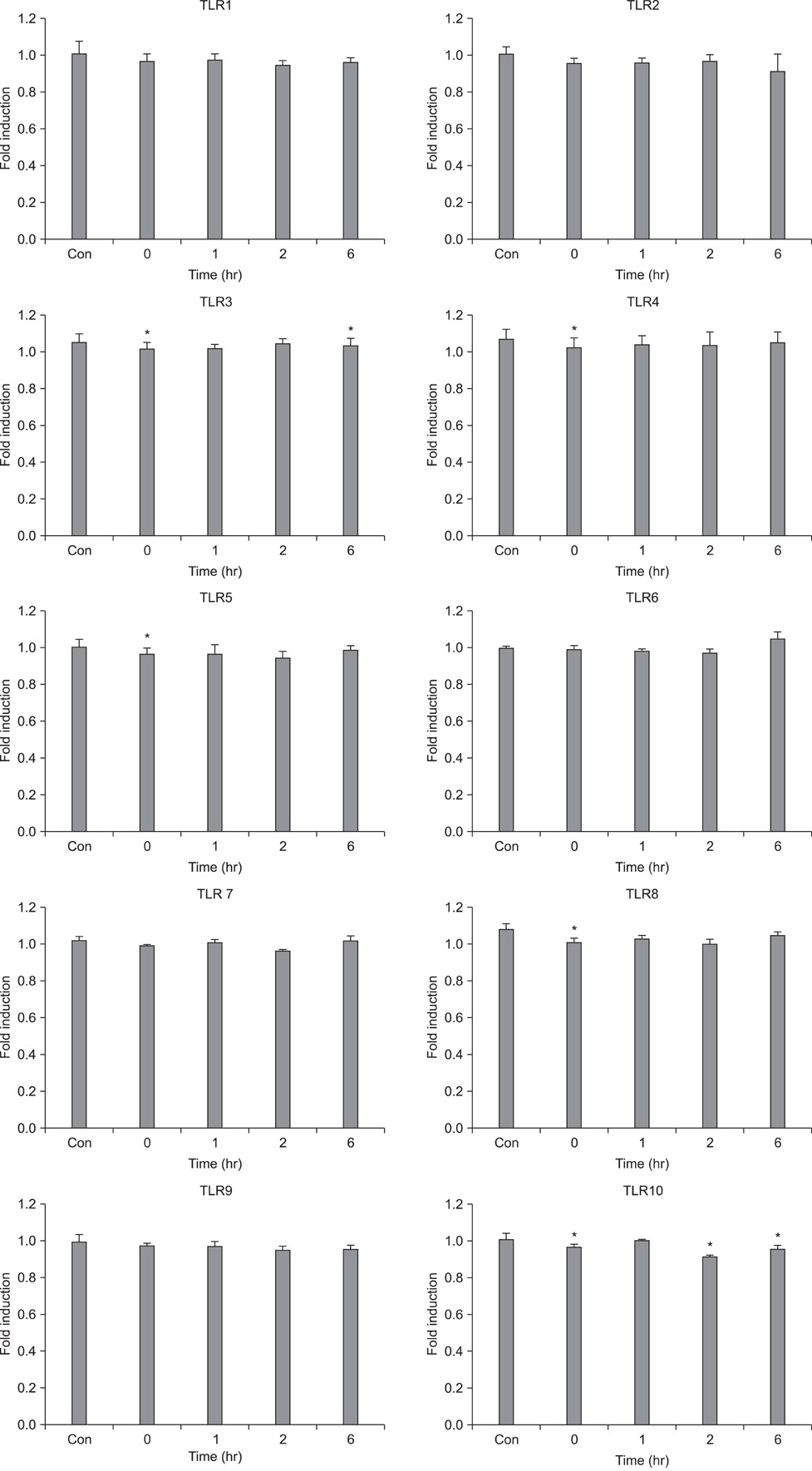
- Fibroblasts are responsible for the synthesis and degradation of various connective tissue components and soluble mediators of extracellular matrix metabolism. Few studies have been conducted concerning the expression of toll-like receptors (TLRs) in fibroblasts until now. This study aimed first to determine the quantitative expression of TLRs 1 to 10 in human skin fibroblasts and secondarily to explore any influence of expression by histamine, which is a well-known factor engaged in dermal inflammation. It was found that all 10 TLRs were expressed in fibroblasts. Interestingly, the expression of TLRs 4, 5, and 10 was increased after 2 and 6 hours of histamine treatment during culture. However, the expression of TLRs 2, 3, 6, 7, 8, and 9 was decreased after 6 hours of histamine treatment. Among the TLRs with a decreasing expression pattern, TLRs 7 and 8 showed a persistent tendency to decrease. All of these changes in TLR expression with histamine treatment were antagonized by treatment with diphenhydramine, a well-known antihistamine. Thus, these results suggest a role of histamine in the early phase of the dermal inflammatory reaction mediated by TLRs.
Keyword
MeSH Terms
Figure
Reference
-
1. Hough LB. Genomics meets histamine receptors: new subtypes, new receptors. Mol Pharmacol. 2001. 59:415–419.
Article2. Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002. 99:12877–12882.
Article3. Kawai T, Akira S. TLR signaling. Semin Immunol. 2007. 19:24–32.
Article4. Talreja J, Kabir MH, B Filla M, Stechschulte DJ, Dileepan KN. Histamine induces Toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to Gram-positive and Gram-negative bacterial cell wall components. Immunology. 2004. 113:224–233.
Article5. Hou YF, Zhou YC, Zheng XX, Wang HY, Fu YL, Fang ZM, et al. Modulation of expression and function of Toll-like receptor 3 in A549 and H292 cells by histamine. Mol Immunol. 2006. 43:1982–1992.
Article6. Kobayashi M, Yoshiki R, Sakabe J, Kabashima K, Nakamura M, Tokura Y. Expression of toll-like receptor 2, NOD2 and dectin-1 and stimulatory effects of their ligands and histamine in normal human keratinocytes. Br J Dermatol. 2009. 160:297–304.
Article7. Proost P, Verpoest S, Van de Borne K, Schutyser E, Struyf S, Put W, et al. Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-gamma in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. J Leukoc Biol. 2004. 75:777–784.
Article8. Kikuchi K, Kadono T, Takehara K. Effects of various growth factors and histamine on cultured keloid fibroblasts. Dermatology. 1995. 190:4–8.
Article9. Falanga V, Soter NA, Altman RD, Kerdel FA. Elevated plasma histamine levels in systemic sclerosis (scleroderma). Arch Dermatol. 1990. 126:336–338.
Article10. Barnes PJ. Histamine receptors in the lung. Agents Actions Suppl. 1991. 33:103–122.
Article11. Hill SJ. Multiple histamine receptors: properties and functional characteristics. Biochem Soc Trans. 1992. 20:122–125.
Article12. Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998. 161:2586–2593.13. Clark RA, Gallin JI, Kaplan AP. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975. 142:1462–1476.
Article14. Raible DG, Schulman ES, DiMuzio J, Cardillo R, Post TJ. Mast cell mediators prostaglandin-D2 and histamine activate human eosinophils. J Immunol. 1992. 148:3536–3542.15. Ogden BE, Hill HR. Histamine regulates lymphocyte mitogenic responses through activation of specific H1 and H2 histamine receptors. Immunology. 1980. 41:107–114.16. Beer DJ, Matloff SM, Rocklin RE. The influence of histamine on immune and inflammatory responses. Adv Immunol. 1984. 35:209–268.
Article17. Li Y, Chi L, Stechschulte DJ, Dileepan KN. Histamine-induced production of interleukin-6 and interleukin-8 by human coronary artery endothelial cells is enhanced by endotoxin and tumor necrosis factor-alpha. Microvasc Res. 2001. 61:253–262.
Article18. Ikawa Y, Shiba K, Ohki E, Mutoh N, Suzuki M, Sato H, et al. Comparative study of histamine H4 receptor expression in human dermal fibroblasts. J Toxicol Sci. 2008. 33:503–508.
Article19. Contoli M, Caramori G, Mallia P, Johnston S, Papi A. Mechanisms of respiratory virus-induced asthma exacerbations. Clin Exp Allergy. 2005. 35:137–145.
Article20. Petricevic B, Wessner B, Sachet M, Vrbanec D, Spittler A, Bergmann M. CL097, a TLR7/8 ligand, inhibits TLR-4-dependent activation of IRAK-M and BCL-3 expression. Shock. 2009. 32:484–490.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of Toll-like Receptors 1, 2, 4, 5, and 7 on Human Melanocytes Modulate Pigmentation
- Neonatal innate immunity and Toll-like receptor
- Toll-like Receptors and Antimicrobial Peptides Expressions of Psoriasis: Correlation with Serum Vitamin D Level
- Toll-Like Receptors and Kidney Diseases
- Calcitriol May Down-Regulate mRNA Over-Expression of Toll-Like Receptor-2 and -4, LL-37 and Proinflammatory Cytokines in Cultured Human Keratinocytes