Korean J Obstet Gynecol.
2010 Mar;53(3):274-281. 10.5468/kjog.2010.53.3.274.
Alendronate enhances osteoblastic differentiation with increased expression of Id-1 and Id-2 in pre-osteoblast cell line, MC3T3-E1
- Affiliations
-
- 1Center for Reproductive Medicine, Busan, Korea.
- 2Department of Obstetrics and Gynecology, Good Moonhwa hospital, Busan, Korea. moonhwas@moonhwa.or.kr
- KMID: 2273853
- DOI: http://doi.org/10.5468/kjog.2010.53.3.274
Abstract
OBJECTIVE
Alendronate, a widely used bisphosphonates, acts to inhibit bone resorption by interfering with the activity of osteoclasts. Recently, it has been reported that alendronate also may increase bone proliferation and osteoblastic differentiation. However, little is known about mechanism of the action of alendronate on osteoblast differentiation, especially in transcription level. Inhibitors of DNA binding/ differentiation (Ids) are helix-loop-helix (HLH) transcription factors and play an important role in BMP-induced osteoblast lineage-specific differentiation. Therefore, this study was aimed to investigate the effect of alendronate on osteoblast differentiation and expression of Id-1 and Id-2.
METHODS
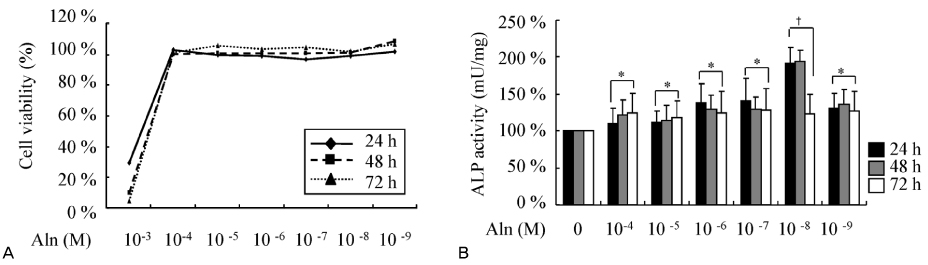
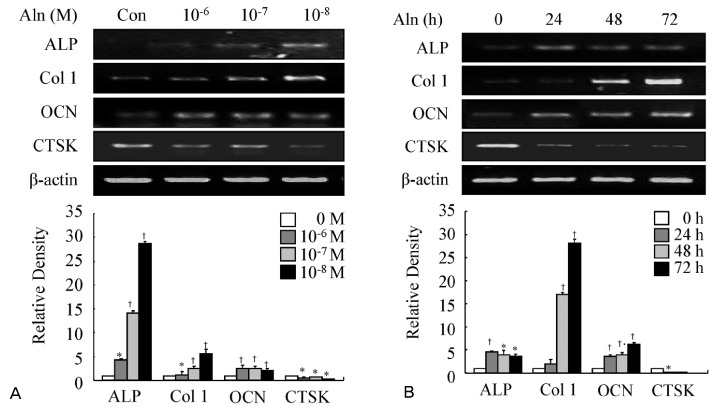
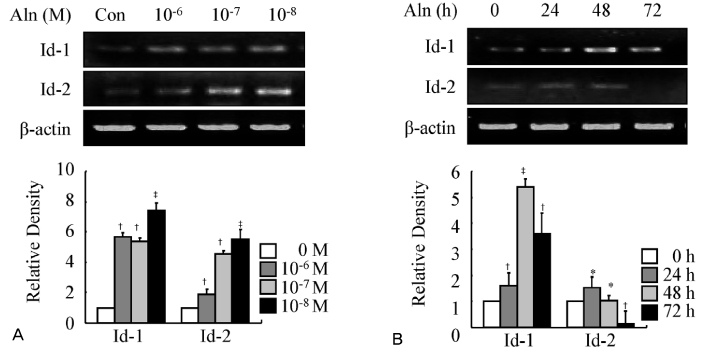
MC3T3-E1, pre-osteoblast cell line, were treated with alendronate of various concentrations (10(-9) M-10(-4) M) and time periods (24, 48 and 72 hours). And then, the effect of alendronate on osteoblast differentiation was examined by alkaline phosphatase (ALP) activity and RT-PCR for osteoblast differentiation markers such as ALP, type 1 collagen (Col 1), and osteocalcin (OCN). The expressions of Id-1 and Id-2 were measured by RT-PCR.
RESULTS
Alendronate treatment increased not only ALP activity, but also expressions of ALP, Col 1, and OCN. Also, alendronate treatment up-regulated the mRNA levels of Id-1 and Id-2 genes. This alendronate-induced osteoblastic differentiation is more effective in lower doses rather than high doses.
CONCLUSION
This study shows that the expression of transcription factor Id-1 and Id-2 was increased in a dose-dependent manner during alendronate-induced osteoblast differentiation.
Keyword
MeSH Terms
-
Alendronate
Alkaline Phosphatase
Antigens, Differentiation
Bone Resorption
Cell Differentiation
Cell Line
Collagen Type I
Delta Sleep-Inducing Peptide
Diphosphonates
DNA
Osteoblasts
Osteocalcin
Osteoclasts
RNA, Messenger
Transcription Factors
Alendronate
Alkaline Phosphatase
Antigens, Differentiation
Collagen Type I
DNA
Delta Sleep-Inducing Peptide
Diphosphonates
Osteocalcin
RNA, Messenger
Transcription Factors
Figure
Reference
-
1. Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002. 8:147–159.
Article2. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003. 423:349–355.
Article3. Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992. 13:66–80.
Article4. Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000. 289:1508–1514.
Article5. Lane JM, Khan SN, O'Connor WJ, Nydick M, Hommen JP, Schneider R, et al. Bisphosphonate therapy in fibrous dysplasia. Clin Orthop. 2001. 382:6–12.
Article6. Devogelaer JP. New uses of bisphosphonates: osteogenesis imperfecta. Curr Opin Pharmacol. 2002. 2:748–753.
Article7. Lehmann HJ, Mouritzen U, Christgau S, Cloos PA, Christiansen C. Effect of bisphosphonates on cartilage turnover assessed with a newly developed assay for collagen type II degradation products. Ann Rheum Dis. 2002. 61:530–533.
Article8. Maksymowych WP. Bisphosphonates for arthritis - a confusing rationale. J Rheumatol. 2003. 30:430–434.9. Fromigué O, Body JJ. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest. 2002. 25:539–546.
Article10. Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004. 25:4105–4115.
Article11. Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008. 473:98–105.
Article12. Malaval L, Liu F, Roche P, Aubin JE. Kinetics of osteoprogenitor proliferation and osteoblast differentiation in vitro. J Cell Biochem. 1999. 74:616–627.
Article13. Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995. 17:2 suppl. 77S–83S.
Article14. Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000. 21:393–411.
Article15. Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, et al. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004. 279:32941–32949.
Article16. Giuliani N, Girasole G, Pedrazzoni M, Passeri G, Gatti C, Passeri M. Alendronate stimulates b-FGF productin and mineralized nodule formation in hman osteoblastic cells and osteoblastogenesis in human bone marrow cultures. J Bone Miner Res. 1995. 10:Suppl. S171.17. Kang Y, Chen CR, Massagué J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell. 2003. 11:915–926.18. Kreider BL, Benezra R, Rovera G, Kadesch T. Inhibition of myeloid differentiation by the helix-loop-helix protein Id. Science. 1992. 255:1700–1702.
Article19. Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003. 13:410–418.
Article21. García-Moreno C, Serrano S, Nacher M, Farré M, Díez A, Mariñoso ML, et al. Effect of alendronate on cultured normal human osteoblasts. Bone. 1998. 22:233–239.
Article22. Sama AA, Khan SN, Myers ER, Huang RC, Cammisa FP, Sandhu HS, et al. High-dose alendronate uncouples osteoclast and osteoblast function: a study in a rat spine pseudarthrosis model. Clin Orthop Relat Res. 2004. 425:135–142.23. Klein BY, Ben-Bassat H, Breuer E, Solomon V, Golomb G. Structurally different bisphosphonates exert opposing effects on alkaline phosphatase and mineralization in marrow osteoprogenitors. J Cell Biochem. 1998. 68:186–194.
Article24. Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, et al. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000. 60:6001–6007.26. Einhorn TA. The cell and fracture healing. Clin Orthop Ralat Res. 1988. 355 suppl. S7–S21.27. Torii Y, Hitomi K, Yamagishi Y, Tsukagoshi N. Demonstration of alkaline phosphatase participation in the mineralization of osteoblasts by antisense RNA approach. Cell Biol Int. 1996. 20:459–464.
Article28. Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003. 85-A:1544–1552.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lactoferrin Constitutively Enhances Differentiation of Osteoblastic MC3T3-E1 Cells in Vitro
- Effects of Ascorbic Acid on Osteoblast Differentiation in MC3T3-E1 Cells
- Effect of Sambucus sieboldiana Extract on the Cell Growth and Extracellular Matrix Formation in Osteoblast Cells
- Zinc modulation of osterix in MC3T3-E1 cells
- Glycyrrhiza uralensis (licorice) extracts increase cell proliferation and bone marker enzyme alkaline phosphatase activity in osteoblastic MC3T3-E1 cells