Cancer Res Treat.
2006 Apr;38(2):112-117.
Augmentation of Sodium Butyrate-induced Apoptosis by Phosphatidylinositol 3-kinase Inhibition in the Human Cervical Cancer Cell-line
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Keimyung University School of Medicine, Daegu, Korea. sdcha@dsmc.or.kr
- 2Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
Abstract
-
PURPOSE: Sodium butyrate (NaBT) is principally a histone deacetylase (HDAC) inhibitor, and it has the potential to arrest HPV-positive carcinoma cells at the G1 to S phase transition of the cell cycle. The aim of study was to determine whether phosphatidylinositol 3-kinase (PI3K) inhibition can enhance the inhibitory effect of NaBT on a human cervical cancer cell line (HeLa).
MATERIALS AND METHODS
Cervical cancer cells (HeLa) were treated with NaBT alone or in combination with the PI3K inhibitors wortmannin or LY294002. Cell viability analysis and FACS analysis were carried out. The expressions of the cell cycle related proteins were evaluated by Western-blot analysis.
RESULTS
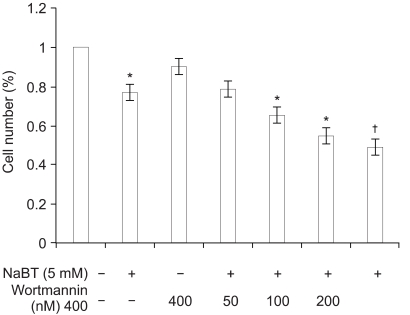
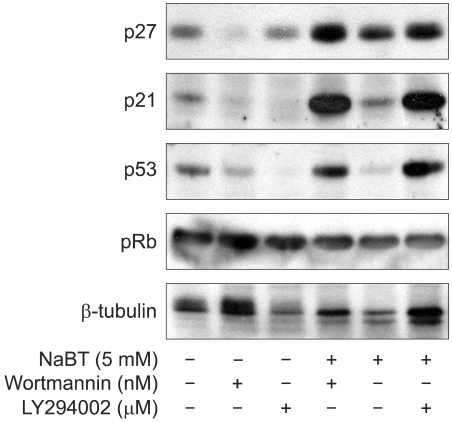
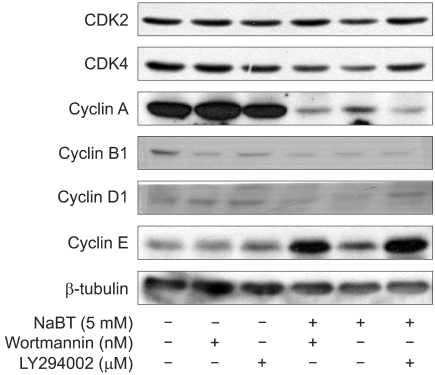
Inhibition of PI3K enhanced NaBT-mediated apoptosis and this decreased the HeLa cell viability. Either wortmannin or LY294002, combined with NaBT, enhanced the activation of caspase 3 and caspase 9, and this enhanced the subsequent cleavage of poly (ADP-ribose) polymerase (PARP). Cervical cancer cells were arrested in the subG1 and G2/M phase, as was detected by FACS analysis. NaBT treatment in combination with PI3K inhibitors showed the increased expression of the CDK inhibitors p21(Cip1/Waf1) and p2(7Kip1), in a p53 dependent manner, and also the increased dephosphorylation of Rb whereas there was a reduction in the expression levels of cyclin A, cyclin D1 and cyclin B1.
CONCLUSION
The results demonstrate that inhibition of PI3K enhances NaBT-mediated cervical cancer cell apoptosis through the activation of the caspase pathway. Moreover, these findings will support future investigation using the PI3K inhibitors in combination with adjuvant treatment for treating carcinoma of the cervix.
MeSH Terms
-
Apoptosis*
Butyric Acid
Caspase 3
Caspase 9
Cell Cycle
Cell Line
Cell Survival
Cervix Uteri
Cyclin A
Cyclin B1
Cyclin D1
Female
HeLa Cells
Histone Deacetylases
Humans*
Phosphatidylinositol 3-Kinase*
Phosphatidylinositols*
S Phase
Sodium*
Uterine Cervical Neoplasms*
Butyric Acid
Caspase 3
Caspase 9
Cyclin A
Cyclin B1
Cyclin D1
Histone Deacetylases
Phosphatidylinositol 3-Kinase
Phosphatidylinositols
Sodium
Figure
Reference
-
1. Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003; 159:283–300. PMID: 12600231.2. Ma BB, Bristow RG, Kim J, Siu LL. Combine-modality treatment of solid tumors using radiotherapy and molecular targeted agents. J Clin Oncol. 2003; 21:2760–2776. PMID: 12860956.3. Roche S, Koegl M, Courtneidge SA. The phosphatidylinositol 3-kinase α is required for DNA synthesis induced by some, but not all, growth factors. Proc Natl Acad Sci USA. 1994; 91:9185–9189. PMID: 8090789.4. Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997; 139:809–815. PMID: 9348296.
Article5. Davidson HW. Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J Cell Biol. 1995; 130:797–805. PMID: 7642698.
Article6. Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999; 11:219–225. PMID: 10209156.
Article7. Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA. 1996; 93:2930–2935. PMID: 8610145.
Article8. Cummings JH. Short-chain fatty acids in the human colon. Gut. 1981; 22:763–779. PMID: 7028579.9. Finzer P, Kuntzen C, Soto U, zur Hausen H, Rosl F. Inhibitors of histone deacetylase arrest cell cycle and induce apoptosis in cervical carcinoma cells circumventing human papillomavirus oncogene expression. Oncogene. 2001; 20:4768–4776. PMID: 11521189.
Article10. Finzer P, Ventz R, Kuntzen C, Seibert N, Soto U, Rosl F. Growth arrest of HPV-positive cells after histone deacetylase inhibition is dependent of E6/E7 oncogene expression. Virology. 2002; 304:265–273. PMID: 12504567.11. Mandal M, Kumar R. Bcl-2 expression regulates sodium butyrate-induced apoptosis in human MCF-7 breast cancer cells. Cell Growth Differ. 1996; 7:311–318. PMID: 8838861.12. Coradini D, Pellizzaro C, Marimpietri D, Abolafio G, Daidone MG. Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Prolif. 2000; 33:139–146. PMID: 10959623.
Article13. Newmark HL, Young CW. Butyrate and phenylacetate as differentiating agents: practical problems and opportunities. J Cell Biochem Suppl. 1995; 22(suppl):247–253. PMID: 8538206.
Article14. Alonio LV, Picconi MA, Dalbert D, Mural J, Bartt O, Bazan G, et al. Ha-ras oncogene mutation associated to progression of papillomavirus induced lesions of uterine cervix. J Clin Virol. 2003; 27:263–269. PMID: 12878090.
Article15. Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001; 70:535–602. PMID: 11395417.
Article16. Benistant C, Chapuis H, Roche S. A specific function for phosphatidylinositol 3-kinase α(p85α-p110α) in cell survival and for phosphatidylinositol 3-kinase β(p85α-p110β) in de novo DNA synthesis of human colon carcinoma cells. Oncogene. 2000; 19:5083–5090. PMID: 11042696.17. Kermorgant S, Aparicio T, Dessirier V, Lewin MJ, Lehy T. Hepatocyte growth factor induces colonic cancer cell invasiveness via enhanced motility and protease overproduction. Evidence for PI3 kinase and PKC involvement. Carcinogenesis. 2001; 22:1035–1042. PMID: 11408346.
Article18. Ma YY, Wei SJ, Lin YC, Lung JC, Chang TC, Whang-Peng J, et al. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000; 25:2739–2744. PMID: 10851074.
Article19. Janson W, Brandner G, Siegel J. Butyrate modulates DNA-damage-induced p53 response by induction of p53-independent differentation and apoptosis. Oncogene. 1997; 15:1395–1406. PMID: 9333015.20. Yih LH, Lee TC. Arsenite induces p53 accumulation through an ATM-dependent pathway in human fibroblasts. Cancer Res. 2000; 60:6346–6352. PMID: 11103796.21. Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Bio. 1996; 16:1722–1733. PMID: 8657148.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment with sodium butyrate and rapamycin inhibit growth of human cervical cancer cells
- Induction of growth inhibition and apoptosis in human endometrial cancer cells by histone deacetylase inhibitors
- The Changes of Expression of Survivin by Butyrate in HCT116 Colon Cancer Cells
- Apoptotic Cell Death Induced by Disruption of Epithelial Cell and Extracellular Matrix Interactions in Mouse Inner Medullary Collection Duct Cells
- Phosphatidylinositol-3-kinase Inhibitor Enhances Nitric Oxide Synthesis and Apoptosis in LPS-Stimulated Macrophages