Ann Dermatol.
2015 Feb;27(1):1-9. 10.5021/ad.2015.27.1.1.
Abnormal B Lymphocyte Activation and Function in Systemic Sclerosis
- Affiliations
-
- 1Department of Dermatology, University of Tokyo Graduate School of Medicine, Tokyo, Japan. ayuyoshi@me.com
- KMID: 2264829
- DOI: http://doi.org/10.5021/ad.2015.27.1.1
Abstract
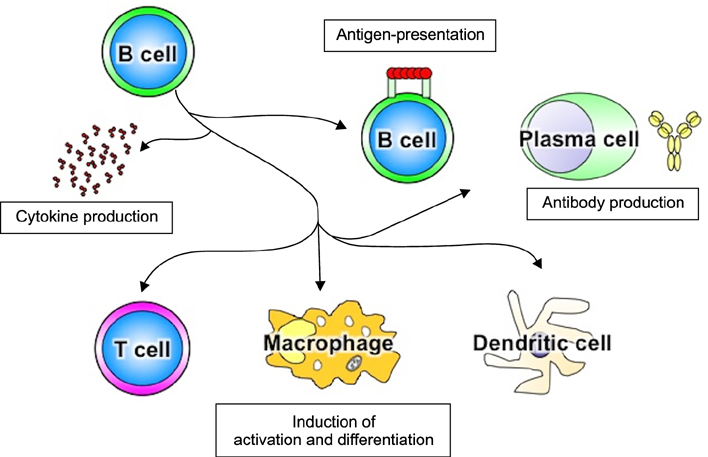
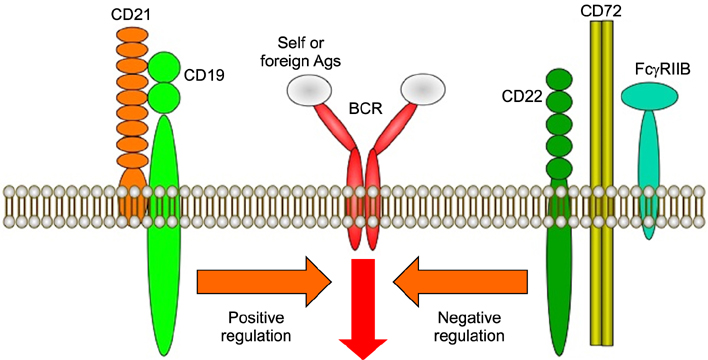
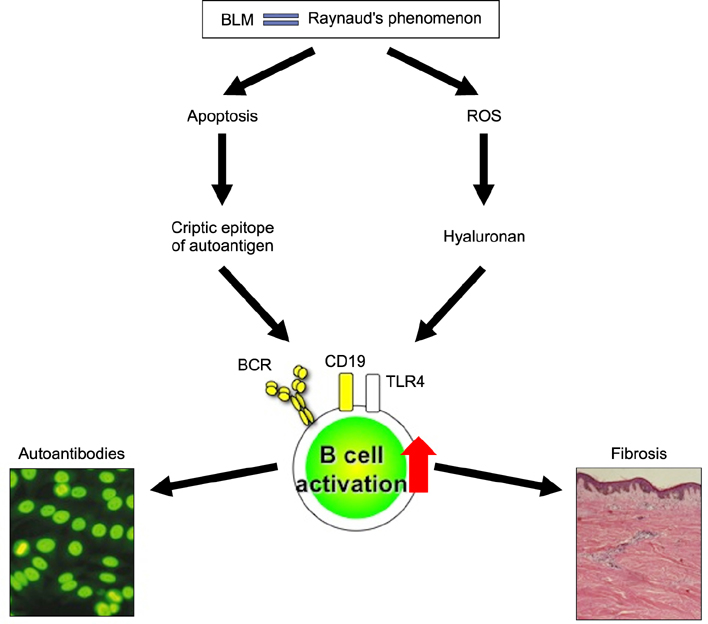
- Systemic sclerosis (SSc) is characterized by tissue fibrosis and autoimmunity. Although the pathogenic relationship between autoimmunity and clinical manifestations of SSc remains unknown, SSc patients display abnormal immune responses including the production of disease-specific autoantibodies. Previous studies have demonstrated that B cells play a critical role in systemic autoimmunity and disease expression through various functions such as induction of the activation of other immune cells in addition to autoantibody production. CD19 is a crucial regulator of B cell activation. Recent studies demonstrated that B cells from SSc patients showed an up-regulated CD19 signaling pathway that induced SSc-specific autoantibody production in SSc mouse models. CD19 transgenic mice lost tolerance for autoantigen and generated autoantibodies spontaneously. B cells from SSc patients exhibited an overexpression of CD19 that induced SSc-specific autoantibody production in transgenic mice. Moreover, SSc patients displayed intrinsic B cell abnormalities characterized by chronic hyper-reactivity of memory B cells, which was possibly due to CD19 overexpression. Similarly, B cells from a tight-skin mouse, a genetic model of SSc, showed augmented CD19 signaling. In bleomycin-induced SSc mouse models, endogenous ligands for toll-like receptor 4 induced by bleomycin stimulated B cells to produce various fibrogenic cytokines and autoantibodies. Remarkably, the loss of CD19 resulted in the inhibition of B cell hyper-reactivity and autoantibody production, which are associated with improvements in fibrosis and a parallel decrease in fibrogenic cytokine production by B cells. Taken together, the findings suggest that altered B cell function may result in tissue fibrosis as well as autoimmunity in SSc.
Keyword
MeSH Terms
Figure
Reference
-
1. LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988; 15:202–205.2. Furst DE, Clements PJ. Hypothesis for the pathogenesis of systemic sclerosis. J Rheumatol Suppl. 1997; 48:53–57.3. Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa A, Muroi E, et al. Elevated serum interleukin-27 levels in patients with systemic sclerosis: association with T cell, B cell and fibroblast activation. Ann Rheum Dis. 2011; 70:194–200.
Article4. Yoshizaki A, Komura K, Iwata Y, Ogawa F, Hara T, Muroi E, et al. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: association with disease severity. J Clin Immunol. 2009; 29:180–189.
Article5. Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma). Rheum Dis Clin North Am. 1996; 22:709–735.
Article6. Hayakawa I, Sato S, Echigo T, Shirasaki F, Hasegawa M, Takehara K. Improvement of skin sclerosis after occurrence of anticentromere antibody in a patient with diffuse cutaneous systemic sclerosis. Clin Rheumatol. 2004; 23:345–347.
Article7. Yoshizaki A, Yanaba K, Ogawa A, Asano Y, Kadono T, Sato S. Immunization with DNA topoisomerase I and Freund's complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheum. 2011; 63:3575–3585.
Article8. Sato S, Fujimoto M, Hasegawa M, Takehara K, Tedder TF. Altered B lymphocyte function induces systemic autoimmunity in systemic sclerosis. Mol Immunol. 2004; 41:1123–1133.
Article9. Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001; 2:764–766.
Article10. Whitfield ML, Finlay DR, Murray JI, Troyanskaya OG, Chi JT, Pergamenschikov A, et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci U S A. 2003; 100:12319–12324.
Article11. Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci U S A. 1996; 93:2264–2271.
Article12. Tedder TF. Introduction: response-regulators of B lymphocyte signaling thresholds provide a context for antigen receptor signal transduction. Semin Immunol. 1998; 10:259–265.
Article13. Tedder TF, Inaoki M, Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997; 6:107–118.
Article14. Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000; 13:277–285.
Article15. Majeti R, Xu Z, Parslow TG, Olson JL, Daikh DI, Killeen N, et al. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000; 103:1059–1070.
Article16. Pan C, Baumgarth N, Parnes JR. CD72-deficient mice reveal nonredundant roles of CD72 in B cell development and activation. Immunity. 1999; 11:495–506.
Article17. Inaoki M, Sato S, Weintraub BC, Goodnow CC, Tedder TF. CD19-regulated signaling thresholds control peripheral tolerance and autoantibody production in B lymphocytes. J Exp Med. 1997; 186:1923–1931.
Article18. Sato S, Ono N, Steeber DA, Pisetsky DS, Tedder TF. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996; 157:4371–4378.19. Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang ML, et al. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996; 5:551–562.
Article20. O'Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996; 274:798–801.21. Painter CJ, Monestier M, Chew A, Bona-Dimitriu A, Kasturi K, Bailey C, et al. Specificities and V genes encoding monoclonal autoantibodies from viable motheaten mice. J Exp Med. 1988; 167:1137–1153.
Article22. Tedder TF, Zhou LJ, Engel P. The CD19/CD21 signal transduction complex of B lymphocytes. Immunol Today. 1994; 15:437–442.
Article23. Tedder TF, Poe JC, Fujimoto M, Haas KM, Sato S. The CD19-CD21 signal transduction complex of B lymphocytes regulates the balance between health and autoimmune disease: systemic sclerosis as a model system. Curr Dir Autoimmun. 2005; 8:55–90.
Article24. Fujimoto M, Poe JC, Hasegawa M, Tedder TF. CD19 regulates intrinsic B lymphocyte signal transduction and activation through a novel mechanism of processive amplification. Immunol Res. 2000; 22:281–298.
Article25. Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995; 3:39–50.
Article26. Rickert RC, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995; 376:352–355.
Article27. Sato S, Steeber DA, Tedder TF. The CD19 signal transduction molecule is a response regulator of B-lymphocyte differentiation. Proc Natl Acad Sci U S A. 1995; 92:11558–11562.
Article28. Sato S, Steeber DA, Jansen PJ, Tedder TF. CD19 expression levels regulate B lymphocyte development: human CD19 restores normal function in mice lacking endogenous CD19. J Immunol. 1997; 158:4662–4669.29. Zhou LJ, Smith HM, Waldschmidt TJ, Schwarting R, Daley J, Tedder TF. Tissue-specific expression of the human CD19 gene in transgenic mice inhibits antigen-independent B-lymphocyte development. Mol Cell Biol. 1994; 14:3884–3894.
Article30. Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J Immunol. 2000; 165:6635–6643.
Article31. Sato S, Fujimoto M, Hasegawa M, Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum. 2004; 50:1918–1927.
Article32. Asano N, Fujimoto M, Yazawa N, Shirasawa S, Hasegawa M, Okochi H, et al. B Lymphocyte signaling established by the CD19/CD22 loop regulates autoimmunity in the tight-skin mouse. Am J Pathol. 2004; 165:641–650.
Article33. Famularo G, Giacomelli R, Alesse E, Cifone MG, Morrone S, Boirivant M, et al. Polyclonal B lymphocyte activation in progressive systemic sclerosis. J Clin Lab Immunol. 1989; 29:59–63.34. Fleischmajer R, Perlish JS, Reeves JR. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977; 20:975–984.
Article35. Fujimoto M, Sato S. B lymphocytes and systemic sclerosis. Curr Opin Rheumatol. 2005; 17:746–751.
Article36. Wang J, Watanabe T. Expression and function of Fas during differentiation and activation of B cells. Int Rev Immunol. 1999; 18:367–379.
Article37. Siracusa LD, McGrath R, Ma Q, Moskow JJ, Manne J, Christner PJ, et al. A tandem duplication within the fibrillin 1 gene is associated with the mouse tight skin mutation. Genome Res. 1996; 6:300–313.
Article38. Saito S, Nishimura H, Phelps RG, Wolf I, Suzuki M, Honjo T, et al. Induction of skin fibrosis in mice expressing a mutated fibrillin-1 gene. Mol Med. 2000; 6:825–836.
Article39. Wallace VA, Kondo S, Kono T, Xing Z, Timms E, Furlonger C, et al. A role for CD4+ T cells in the pathogenesis of skin fibrosis in tight skin mice. Eur J Immunol. 1994; 24:1463–1466.
Article40. Kodera T, McGaha TL, Phelps R, Paul WE, Bona CA. Disrupting the IL-4 gene rescues mice homozygous for the tight-skin mutation from embryonic death and diminishes TGF-beta production by fibroblasts. Proc Natl Acad Sci U S A. 2002; 99:3800–3805.
Article41. Zhu H, Bona C, McGaha TL. Polymorphisms of the TGF-beta1 promoter in tight skin (TSK) mice. Autoimmunity. 2004; 37:51–55.
Article42. Saito E, Fujimoto M, Hasegawa M, Komura K, Hamaguchi Y, Kaburagi Y, et al. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J Clin Invest. 2002; 109:1453–1462.
Article43. Hasegawa M, Fujimoto M, Takehara K, Sato S. Pathogenesis of systemic sclerosis: altered B cell function is the key linking systemic autoimmunity and tissue fibrosis. J Dermatol Sci. 2005; 39:1–7.
Article44. Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, et al. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999; 112:456–462.
Article45. Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006; 297:333–344.
Article46. Matsushita T, Fujimoto M, Hasegawa M, Matsushita Y, Komura K, Ogawa F, et al. BAFF antagonist attenuates the development of skin fibrosis in tight-skin mice. J Invest Dermatol. 2007; 127:2772–2780.
Article47. Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, et al. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol. 2006; 169:954–966.
Article48. Arnett FC, Cho M, Chatterjee S, Aguilar MB, Reveille JD, Mayes MD. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis Rheum. 2001; 44:1359–1362.
Article49. Yoshizaki A, Iwata Y, Komura K, Ogawa F, Hara T, Muroi E, et al. CD19 regulates skin and lung fibrosis via Toll-like receptor signaling in a model of bleomycin-induced scleroderma. Am J Pathol. 2008; 172:1650–1663.
Article50. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002; 20:197–216.
Article51. Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006; 6:823–835.
Article52. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005; 5:331–342.
Article53. Yoshizaki A, Iwata Y, Komura K, Hara T, Ogawa F, Muroi E, et al. Clinical significance of serum hyaluronan levels in systemic sclerosis: association with disease severity. J Rheumatol. 2008; 35:1825–1829.54. Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999; 6:6–12.
Article55. Casiano CA, Martin SJ, Green DR, Tan EM. Selective cleavage of nuclear autoantigens during CD95 (Fas/APO-1)-mediated T cell apoptosis. J Exp Med. 1996; 184:765–770.
Article56. Sgonc R, Gruschwitz MS, Dietrich H, Recheis H, Gershwin ME, Wick G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996; 98:785–792.
Article57. Yamamoto T, Nishioka K. Possible role of apoptosis in the pathogenesis of bleomycin-induced scleroderma. J Invest Dermatol. 2004; 122:44–50.
Article58. Inghilleri S, Morbini P, Oggionni T, Barni S, Fenoglio C. In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol. 2006; 125:661–669.
Article59. Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, et al. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005; 6:11.
Article60. Yoshizaki A, Yanaba K, Ogawa A, Iwata Y, Ogawa F, Takenaka M, et al. The specific free radical scavenger edaravone suppresses fibrosis in the bleomycin-induced and tight skin mouse models of systemic sclerosis. Arthritis Rheum. 2011; 63:3086–3097.
Article61. Ogawa F, Shimizu K, Muroi E, Hara T, Hasegawa M, Takehara K, et al. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology (Oxford). 2006; 45:815–818.
Article62. Simonini G, Pignone A, Generini S, Falcini F, Cerinic MM. Emerging potentials for an antioxidant therapy as a new approach to the treatment of systemic sclerosis. Toxicology. 2000; 155:1–15.
Article63. Mendoza G, Alvarez AI, Pulido MM, Molina AJ, Merino G, Real R, et al. Inhibitory effects of different antioxidants on hyaluronan depolymerization. Carbohydr Res. 2007; 342:96–102.
Article64. Freitas JP, Filipe P, Emerit I, Meunier P, Manso CF, Guerra Rodrigo F. Hyaluronic acid in progressive systemic sclerosis. Dermatology. 1996; 192:46–49.
Article65. Neudecker BA, Stern R, Connolly MK. Aberrant serum hyaluronan and hyaluronidase levels in scleroderma. Br J Dermatol. 2004; 150:469–476.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of hypoparathyroidism and hypothyroidism in systemic sclerosis
- A Case of Systemic Sclerosis with Coincidental Lung Cancer
- Effect of Raynaud's Phenomenon on Espohageal Motility
- A Case of SLE-systemic Sclerosis Overlap Syndrome Complicated with Nephrotic Syndrome
- A Case of Systemic Sclerosis in a Child