Ann Dermatol.
2015 Apr;27(2):121-127. 10.5021/ad.2015.27.2.121.
Retinoic Acid Promotes Interleukin-4 Plasmid-Dimethylsulfoxide Topical Transdermal Delivery for Treatment of Psoriasis
- Affiliations
-
- 1State Key Laboratory of Biotherapy, West China Hospital, West China Medical School, Sichuan University, Chengdu, China. lijionghh@sohu.com
- KMID: 2264799
- DOI: http://doi.org/10.5021/ad.2015.27.2.121
Abstract
- BACKGROUND
Psoriasis is an autoimmune disease that is caused by a shift in the Th1/Th2 balance toward Th1-dominant immunity. It has been established as an effective treatment to counteract psoriasis by subcutaneous injection of recombinant interleukin (IL)-4, and IL-4 gene therapy by topical transdermal penetration has shown its antipsoriatic effect in mice. Retinoic acid (RA) and dimethylsulfoxide can increase the efficiency of gene transfection in the topical transdermal delivery system.
OBJECTIVE
We investigated whether RA could improve anti-psoriasis efficiency using IL-4 expression plasmid pORF-mIL-4 (pIL-4) via transdermal delivery system in K14-vascular endothelial growth (K14-VEGF) factor transgenic mice.
METHODS
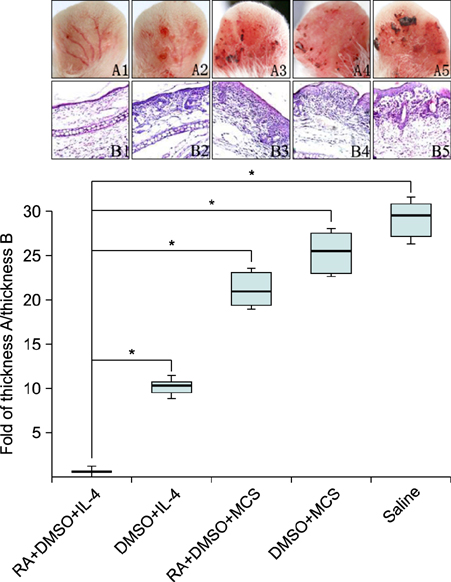
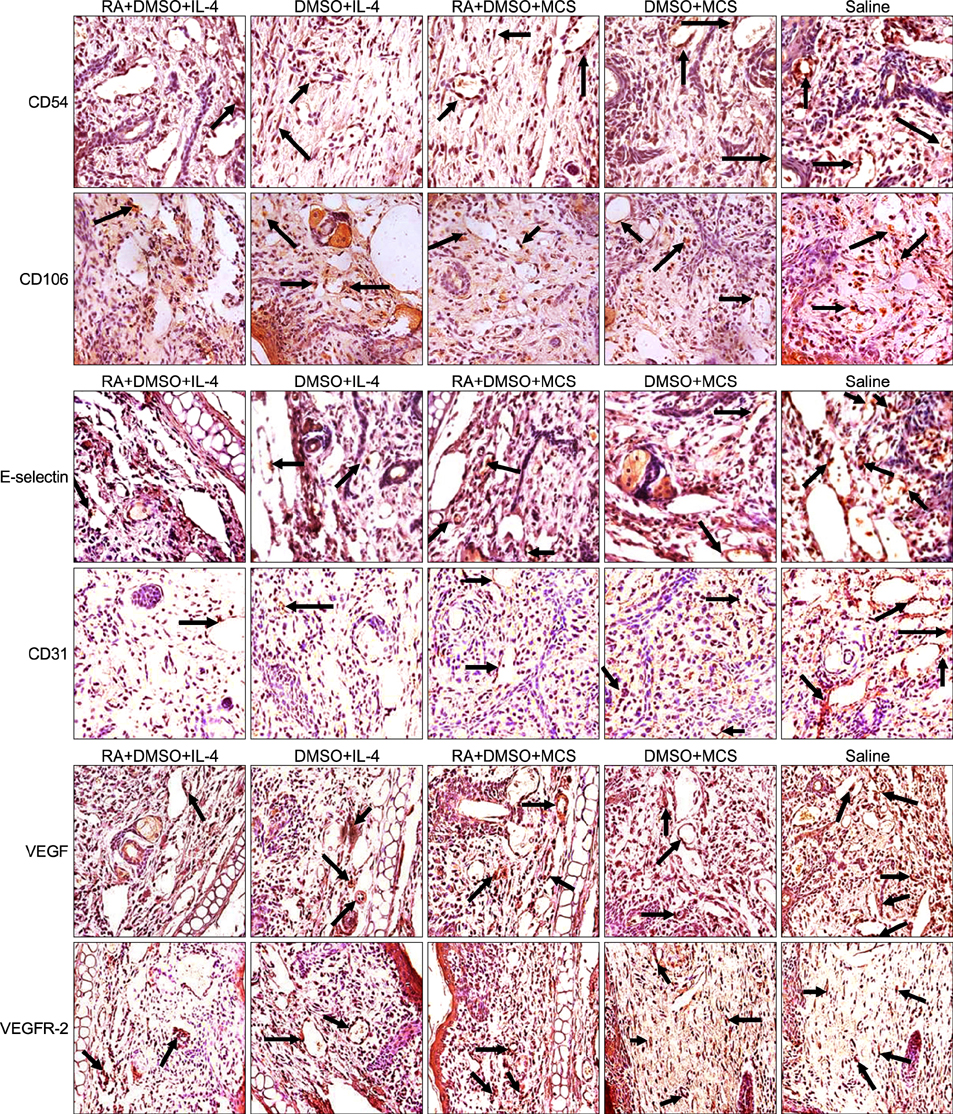
After pretreatment with RA, plasmid pIL-4 in 10% dimethylsulfoxide was applied to the ear skin by topical transdermal penetration. Hematoxylin- eosin staining and immunohistochemistry were performed with ear samples to evaluate anti-psoriasis efficiency in mice.
RESULTS
The psoriasis pathological features were relieved and psoriasis-associated factors were significantly reduced.
CONCLUSION
Our results reveal that topical application of pIL-4 in dimethylsulfoxide by transdermal delivery with RA pretreatment can improve psoriasis significantly.
MeSH Terms
Figure
Reference
-
1. Reich A, Szepietowski J. Genetic and immunological aspects of the pathogenesis of psoriasis. Wiad Lek. 2007; 60:270–276.2. Boniface K, Lecron JC, Bernard FX, Dagregorio G, Guillet G, Nau F, et al. Keratinocytes as targets for interleukin-10-related cytokines: a putative role in the pathogenesis of psoriasis. Eur Cytokine Netw. 2005; 16:309–319.3. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007; 445:866–873.
Article4. Pastore S, Mascia F, Mariotti F, Dattilo C, Girolomoni G. Chemokine networks in inflammatory skin diseases. Eur J Dermatol. 2004; 14:203–208.5. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005; 6:1123–1132.
Article6. Ghoreschi K, Thomas P, Breit S, Dugas M, Mailhammer R, van Eden W, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003; 9:40–46.
Article7. Ren X, Li J, Zhou X, Luo X, Huang N, Wang Y, et al. Recombinant murine interleukin 4 protein therapy for psoriasis in a transgenic VEGF mouse model. Dermatology. 2009; 219:232–238.
Article8. Chen X, Zhang Y, Liu C, Zhang Y, Zhou X, Zhou T, et al. Retinoic acid and dimethyl sulfoxide promote efficient delivery of transgenes to mouse skin by topically transdermal penetration. Drug Deliv. 2010; 17:385–390.
Article9. Lee VH. Advanced drug delivery reviews: cornerstone in the stimulation and dissemination of innovative drug delivery research. Adv Drug Deliv Rev. 2004; 56:1–2.
Article10. Zhang Y, Li J, Liu CY, Zhou XK, Qiu J, Zhang YB, et al. A novel transdermal plasmid-dimethylsulfoxide delivery technique for treatment of psoriasis. Dermatology. 2010; 221:84–92.
Article11. Chen Q, Ross AC. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp Cell Res. 2004; 297:68–81.
Article12. Karlsson T, Virtanen M, Sirsjö A, Rollman O, Vahlquist A, Törmä H. Topical retinoic acid alters the expression of cellular retinoic acid-binding protein-I and cellular retinoic acid-binding protein-II in non-lesional but not lesional psoriatic skin. Exp Dermatol. 2002; 11:143–152.
Article13. Domashenko A, Gupta S, Cotsarelis G. Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat Biotechnol. 2000; 18:420–423.
Article14. Park KM, Kang HC, Cho JK, Chung IJ, Cho SH, Bae YH, et al. All-trans-retinoic acid (ATRA)-grafted polymeric gene carriers for nuclear translocation and cell growth control. Biomaterials. 2009; 30:2642–2652.
Article15. Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003; 102:161–168.
Article16. Baker BS, Brent L, Valdimarsson H, Powles AV, al-Imara L, Walker M, et al. Is epidermal cell proliferation in psoriatic skin grafts on nude mice driven by T-cell derived cytokines? Br J Dermatol. 1992; 126:105–110.
Article17. Schön MP, Krahn T, Schön M, Rodriguez ML, Antonicek H, Schultz JE, et al. Efomycine M, a new specific inhibitor of selectin, impairs leukocyte adhesion and alleviates cutaneous inflammation. Nat Med. 2002; 8:366–372.
Article18. Grailer JJ, Kodera M, Steeber DA. L-selectin: role in regulating homeostasis and cutaneous inflammation. J Dermatol Sci. 2009; 56:141–147.
Article19. Nelson AA, Pearce DJ, Fleischer AB, Balkrishnan R, Feldman SR. New treatments for psoriasis: which biologic is best? J Dermatolog Treat. 2006; 17:96–107.
Article20. Pariser DM, Bagel J, Gelfand JM, Korman NJ, Ritchlin CT, Strober BE, et al. National Psoriasis Foundation. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007; 143:239–242.
Article21. Liu Y, Helms C, Liao W, Zaba LC, Duan S, Gardner J, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008; 4:e1000041.
Article22. Gonze D, Halloy J, Leloup JC, Goldbeter A. Stochastic models for circadian rhythms: effect of molecular noise on periodic and chaotic behaviour. C R Biol. 2003; 326:189–203.
Article23. Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999; 93:580–589.
Article24. Sigmundsdottir H, Gudjonsson JE, Valdimarsson H. The effects of ultraviolet B treatment on the expression of adhesion molecules by circulating T lymphocytes in psoriasis. Br J Dermatol. 2003; 148:996–1000.
Article25. Eşrefoğlu M, Gül M, Seyhan M. Ultrastructural findings and tumor necrosis factor-alpha and intercellular adhesion molecule-1 expression in psoriasis patients before and after oral cyclosporin A therapy. Ultrastruct Pathol. 2006; 30:95–102.
Article26. Creamer D, Allen MH, Sousa A, Poston R, Barker JN. Localization of endothelial proliferation and microvascular expansion in active plaque psoriasis. Br J Dermatol. 1997; 136:859–865.
Article27. Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009; 90:232–248.
Article28. Bovenschen HJ, Otero ME, Langewouters AM, van Vlijmen-Willems IM, van Rens DW, Seyger MM, et al. Oral retinoic acid metabolism blocking agent Rambazole for plaque psoriasis: an immunohistochemical study. Br J Dermatol. 2007; 156:263–270.
Article29. Geria AN, Scheinfeld NS. Talarozole, a selective inhibitor of P450-mediated all-trans retinoic acid for the treatment of psoriasis and acne. Curr Opin Investig Drugs. 2008; 9:1228–1237.30. Mrass P, Rendl M, Mildner M, Gruber F, Lengauer B, Ballaun C, et al. Retinoic acid increases the expression of p53 and proapoptotic caspases and sensitizes keratinocytes to apoptosis: a possible explanation for tumor preventive action of retinoids. Cancer Res. 2004; 64:6542–6548.
Article31. Colucci M, Maione F, Bonito MC, Piscopo A, Di Giannuario A, Pieretti S. New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol Res. 2008; 57:419–425.
Article32. Li J, Chen X, Liu Z, Yue Q, Liu H. Expression of Th17 cytokines in skin lesions of patients with psoriasis. J Huazhong Univ Sci Technolog Med Sci. 2007; 27:330–332.
Article33. Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010; 130:1373–1383.
Article34. Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009; 129:79–88.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of transdermal exposure of phthalates in children's products
- The Therapeutic Effect of Oral Retinoid (Ro - 10 - 9359) on Psoriasis Vulgaris
- Immunomodulatory Effects of Vitamin D Analogues in Psoriatic Skin
- Alternating Topical Treatment with Perinoic Acid And Retinoid (Ro 11 - 1430) on Acne Vulgaris: therapeutic effect and side reactions
- The Effect of Combination Treatment with Ustekinumab and Topical Agents in Korean Patients with Moderate-to-severe Psoriasis: A Retrospective Study of 30 Patients through 5 Years of Follow Up