Allergy Asthma Immunol Res.
2010 Jan;2(1):41-47. 10.4168/aair.2010.2.1.41.
Chlamydophila pneumoniae enhances secretion of VEGF, TGF-beta and TIMP-1 from human bronchial epithelial cells under Th2 dominant microenvironment
- Affiliations
-
- 1Department of Allergy, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. yscho@amc.seoul.kr
- 2Asan Institute for Life Science, Seoul, Korea.
- KMID: 2260418
- DOI: http://doi.org/10.4168/aair.2010.2.1.41
Abstract
- PURPOSE
Chlamydophila pneumoniae infection in the airways is thought to be associated with the pathogenesis of asthma, especially in non-atopic severe asthma with irreversible airway obstruction that may be related to airway remodeling. Here, we investigated whether C. pneumoniae infection enhances the secretion of critical chemical mediators for airway remodeling, such as VEGF, TGF-beta, and TIMP-1, in human bronchial epithelial cells (BECs) in a Th2-dominant microenvironment.
METHODS
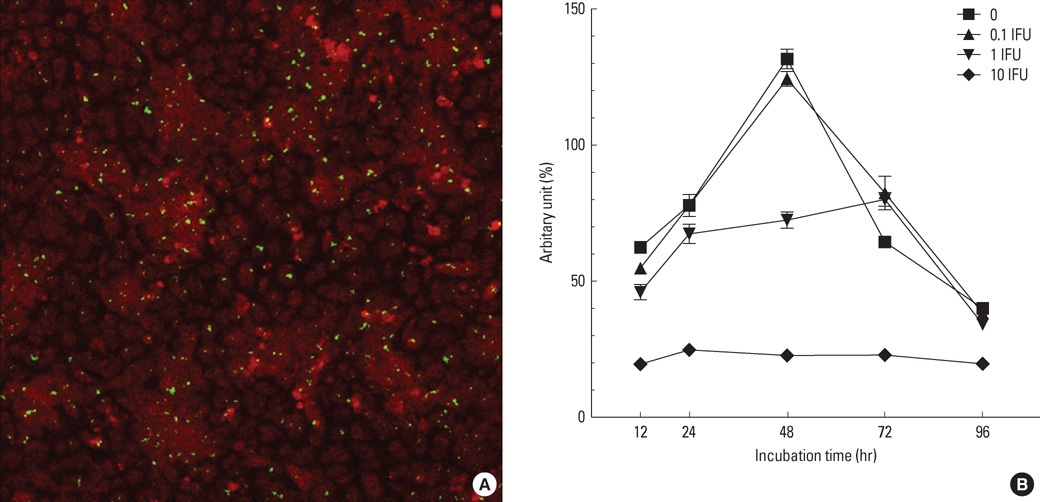
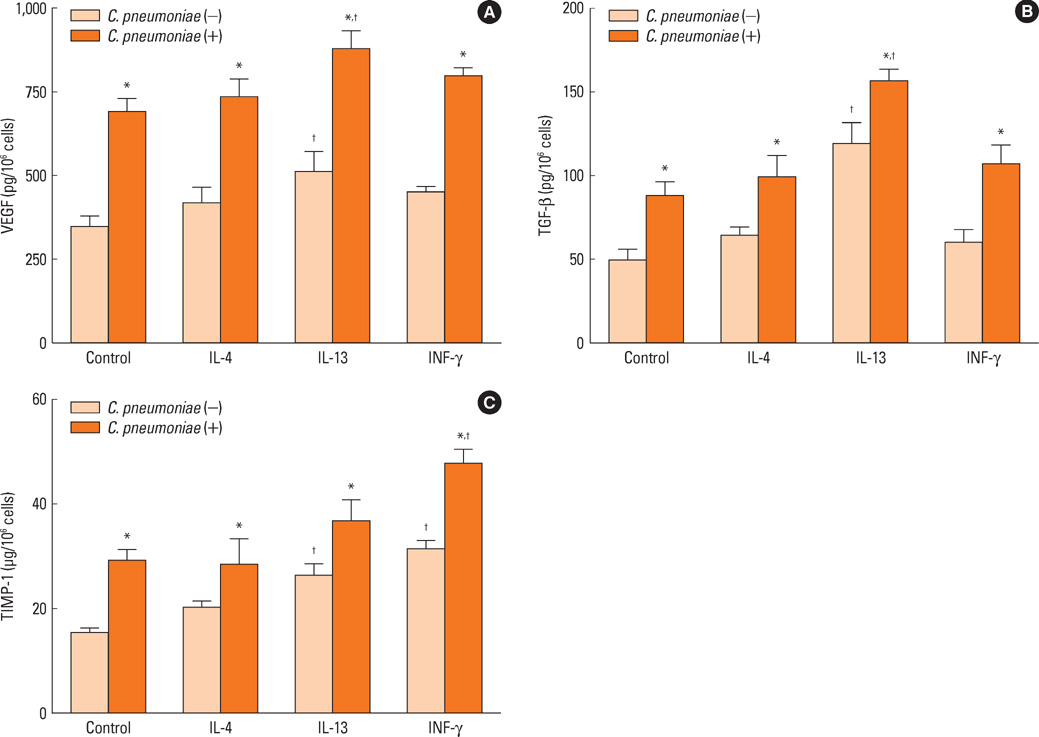
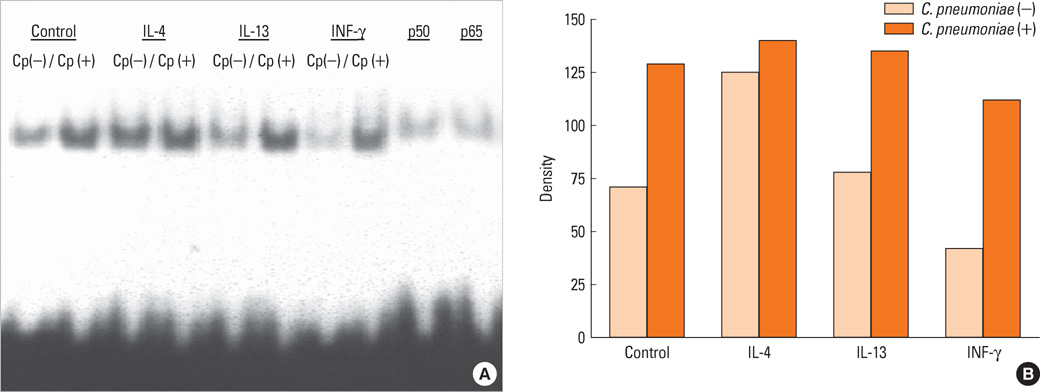
Human bronchial epithelial cells (BEAS-2B cells) were infected with C. pneumoniae strain TW183 and cultured in both a Th1-dominant microenvironment with INF-gamma and a Th2-dominant microenvironment with IL-4 or IL-13 added to the culture medium. The VEGF, TGF-beta, and TIMP-1 levels in the culture supernatants were measured using enzyme-linked immunosorbent assays (ELISA). The activation of NF-kappaB in each experimental condition was determined using an electrophoretic mobility shift assay.
RESULTS
Chlamydophila pneumoniae-infected BECs showed enhanced secretion of VEGF, TGF-beta, and TIMP-1 compared with non-infected BECs. The levels of cytokines secreted from BECs were increased more when IL-13 was added to the culture medium. C. pneumoniae-infected BECs also showed increased NF-kappaB activation.
CONCLUSIONS
These results suggest that C. pneumoniae plays a role in the pathogenesis of airway remodeling in asthma, revealing a Th2-dominant immune response. Further studies are required to clarify the precise mechanism of C. pneumoniae infection in airway remodeling.
Keyword
MeSH Terms
-
Airway Obstruction
Airway Remodeling
Asthma
Chlamydial Pneumonia
Chlamydophila
Chlamydophila pneumoniae
Cytokines
Electrophoretic Mobility Shift Assay
Enzyme-Linked Immunosorbent Assay
Epithelial Cells
Humans
Interleukin-13
Interleukin-4
NF-kappa B
Pneumonia
Sprains and Strains
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinases
Transforming Growth Factor beta
Vascular Endothelial Growth Factor A
Cytokines
Interleukin-13
Interleukin-4
NF-kappa B
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinases
Transforming Growth Factor beta
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Grayston JT, Campbell LA, Kuo CC, Mordhorst CH, Saikku P, Thom DH, Wang SP. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990. 161:618–625.2. Kuo CC, Jackson LA, Campbell LA, Grayston JT. Chlamydia pneumoniae (TWAR). Clin Microbiol Rev. 1995. 8:451–461.3. Cook PJ, Davies P, Tunnicliffe W, Ayres JG, Honeybourne D, Wise R. Chlamydia pneumoniae and asthma. Thorax. 1998. 53:254–259.4. Falck G, Gnarpe J, Hansson L-O, Svardsudd K, Gnarpe H. Comparison of Individuals With and Without Specific IgA Antibodies to Chlamydia pneumoniae: Respiratory Morbidity and the Metabolic Syndrome. Chest. 2002. 122:1587–1593.5. Von Hertzen L, Vasankari T, Liippo K, Wahlström E, Puolakkainen M. Chlamydia pneumoniae and Severity of Asthma. Scand J Infect Dis. 2002. 34:22–27.6. Betsou F, Sueur JM, Orfila J. Anti-Chlamydia pneumoniae heat shock protein 10 antibodies in asthmatic adults. FEMS Immunol Med Microbiol. 2003. 35:107–111.7. Miyashita N, Kubota Y, Nakajima M, Niki Y, Kawane H, Matsushima T. Chlamydia pneumoniae and exacerbations of asthma in adults. Ann Allergy Asthma Immunol. 1998. 80:405–409.8. Esposito S, Blasi F, Arosio C, Fioravanti L, Fagetti L, Droghetti R, Tarsia P, Allegra L, Principi N. Importance of acute Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur Respir J. 2000. 16:1142–1146.9. Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991. 266:225–230.10. Pasternack R, Huhtala H, Karjalainen J. Chlamydophila (Chlamydia) pneumoniae serology and asthma in adults: A longitudinal analysis. J Allergy Clin Immunol. 2005. 116:1123–1128.11. ten Brinke A. Risk factors associated with irreversible airflow limitation in asthma. Curr Opin Allergy Clin Immunol. 2008. 8:63–69.12. Rödel J, Woytas M, Groh A, Schmidt KH, Hartmann M, Lehmann M, Straube E. Production of Basic Fibroblast Growth Factor and Interleukin 6 by Human Smooth Muscle Cells following Infection with Chlamydia pneumoniae. Infect Immun. 2000. 68:3635–3641.13. Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999. 103:571–577.14. Kaukoranta-Tolvanen SSE, Teppo AM, Laitinen K, Saikku P, Linnavuori K, Leinonen M. Growth of Chlamydia pneumoniaein cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb Pathog. 1996. 21:215–221.15. Byrne GI, Ojcius DM. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol. 2004. 2:802–808.16. Caspar-Bauguil S, Puissant B, Nazzal D, Lefèvre JC, Thomsen M, Salvayre R, Benoist H. Chlamydia pneumoniae Induces Interleukin-10 Production that Down-Regulates Major Histocompatibility Complex Class I Expression. J Infect Dis. 2000. 182:1394–1401.17. Cho YS, Kim TB, Lee TH, Moon KA, Lee J, Kim YK, Lee KY, Moon HB. Chlamydia pneumoniae infection enhances cellular proliferation and reduces steroid responsiveness of human peripheral blood mononuclear cells via a tumor necrosis factor-α-dependent pathway. Clin Exp Allergy. 2005. 35:1625–1631.18. Jahn HU, Krüll M, Wuppermann FN, Klucken AC, Rosseau S, Seybold J, Hegemann JH, Jantos CA, Suttorp N. Infection and activation of airway epithelial cells by Chlamydia pneumoniae. J Infect Dis. 2000. 182:1678–1687.19. Yang J, Hooper WC, Phillips DJ, Tondella ML, Talkington DF. Induction of Proinflammatory Cytokines in Human Lung Epithelial Cells during Chlamydia pneumoniae Infection. Infect Immun. 2003. 71:614–620.20. Kim TB, Moon KA, Lee KY, Park CS, Bae YJ, Moon HB, Cho YS. Chlamydophila pneumoniae triggers release of CCL20 and vascular endothelial growth factor from human bronchial epithelial cells through enhanced intracellular oxidative stress and MAPK activation. J Clin Immunol. 2009. 29:629–636.21. Cho YS, Kim TB, Lee TH, Moon KA, Lee J, Kim YK, Lee KY, Moon HB. Chlamydia pneumoniae infection enhances cellular proliferation and reduces steroid responsiveness of human peripheral blood mononuclear cells via a tumor necrosis factor-α-dependent pathway. Clin Exp Allergy. 2005. 35:1625–1631.22. Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989. 86:2336–2340.23. Jeffery PK. Remodeling and Inflammation of Bronchi in Asthma and Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc. 2004. 1:176–183.24. Beatty WL, Morrison RP, Byrne GI. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994. 58:686–699.25. Wen FQ, Liu X, Manda W, Terasaki Y, Kobayashi T, Abe S, Fang Q, Ertl R, Manouilova L, Rennard SI. TH2 Cytokine-enhanced and TGF-β-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-γ and corticosteroids. J Allergy Clin Immunol. 2003. 111:1307–1318.26. Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway Exposure Levels of Lipopolysaccharide Determine Type 1 versus Type 2 Experimental Asthma. J Immunol. 2007. 178:5375–5382.27. Magnan AO, Mély LG, Camilla CA, Badier MM, Montero-Julian FA, Guillot CM, Casano BB, Prato SJ, Fert V, Bongrand P, Vervloet D. Assessment of the Th1/Th2 Paradigm in Whole Blood in Atopy and Asthma. Increased IFN-γ-producing CD8+ T Cells in Asthma. Am J Respir Crit Care Med. 2000. 161:1790–1796.28. Bosse M, Chakir J, Rouabhia M, Boulet LP, Audette M, Laviolette M. Serum Matrix Metalloproteinase-9: Tissue Inhibitor of Metalloproteinase-1 Ratio Correlates with Steroid Responsiveness in Moderate to Severe Asthma. Am J Respir Crit Care Med. 1999. 159:596–602.29. Matsumoto H, Niimi A, Takemura M, Ueda T, Minakuchi M, Tabuena R, Chin K, Mio T, Ito Y, Muro S, Hirai T, Morita S, Fukuhara S, Mishima M. Relationship of airway wall thickening to an imbalance between matrix metalloproteinase-9 and its inhibitor in asthma. Thorax. 2005. 60:277–281.30. Dechend R, Maass M, Gieffers J, Dietz R, Scheidereit C, Leutz A, Gulba DC. Chlamydia pneumoniae Infection of Vascular Smooth Muscle and Endothelial Cells Activates NF-κB and Induces Tissue Factor and PAI-1 Expression: A Potential Link to Accelerated Arteriosclerosis. Circulation. 1999. 100:1369–1373.31. Krüll M, Bockstaller P, Wuppermann FN, Klucken AC, Mühling J, Schmeck B, Seybold J, Walter C, Maass M, Rosseau S, Hegemann JH, Suttorp N, Hippenstiel S. Mechanisms of Chlamydophila pneumoniae-Mediated GM-CSF Release in Human Bronchial Epithelial Cells. Am J Respir Cell Mol Biol. 2006. 34:375–382.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dexamethasone Attenuates PDGF- and TGF-beta-enhanced Vascular Endothelial Growth Factor Production in Cultured Human Bronchial Smooth Muscle Cells
- The Effect of Cigarette Smoking on the Expression of MMP-9, TIMP-1 and VEGF in Nasal Polyp Epithelial Cells and Fibroblasts
- Effects of Transforming Growth Factor-beta on Proliferation, Collagen synthesis, Migration and Metalloproteinase Secretion of Human Retinal Pigment Epithelial Cells
- Transforming growth factor-beta promoted vascular endothelial growth factor release by human lung fibroblasts
- Mycoplasma pneumoniae-induced production of proasthmatic mediators in airway epithelium