Korean J Hematol.
2008 Sep;43(3):150-158. 10.5045/kjh.2008.43.3.150.
Comparison of Immune Responses Induced by Deferoxamine and Deferasirox
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea. dcjeong@catholic.ac.kr
- 2Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Biochemistry, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2252181
- DOI: http://doi.org/10.5045/kjh.2008.43.3.150
Abstract
-
BACKGROUND: The iron chelating agents (ICA) have various biological effects besides iron chelation. We investigated the immunomodulatory effects of Deferasirox (DFS) compared to Deferoxamine (DFO).
METHODS
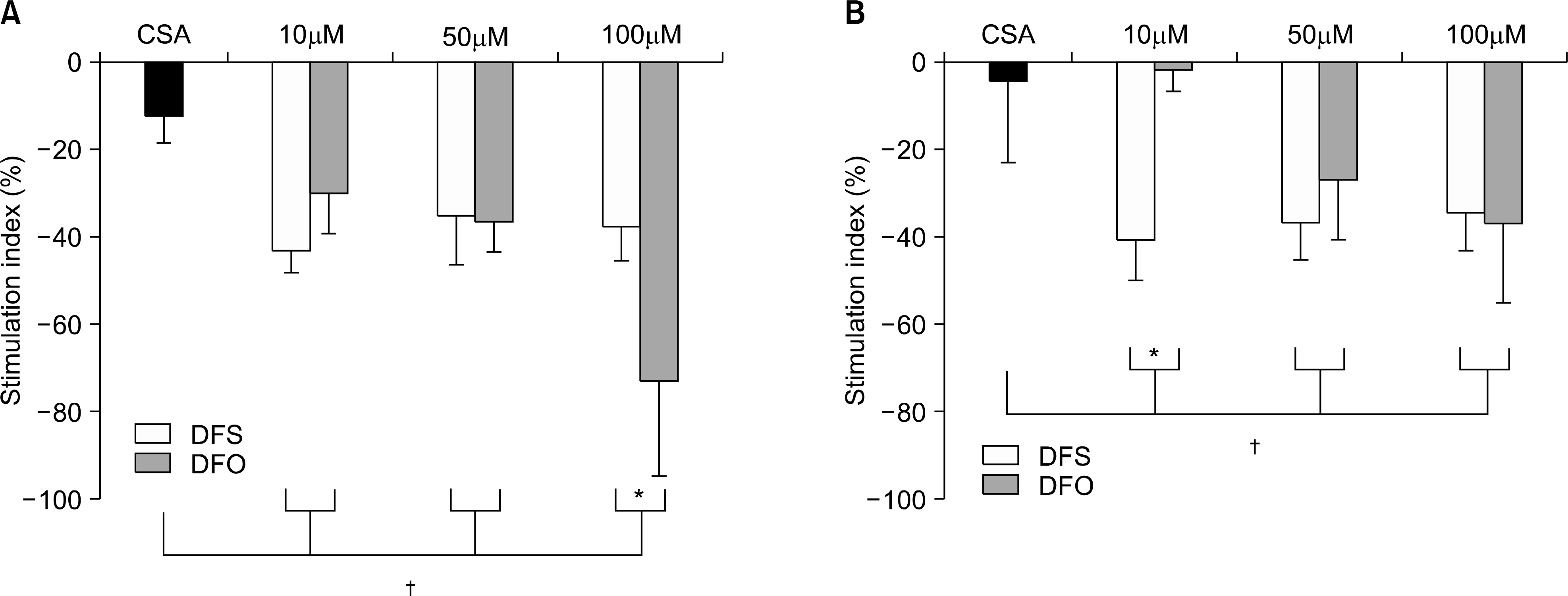
Spleen cells (SP) were obtained from 5 week-old C57/BL6 (H-2(b)). The cytotoxicity of ICAs was examined using the CCK8 method. For the cell proliferation assay, SP were cultured with irradiated in addition to 10, 50, 100micrometer of DFS or DFO and 200ng/mL of cyclosporin A (CSA). Cytokines and nitrite levels were evaluated from supernatants by ELISA.
RESULTS
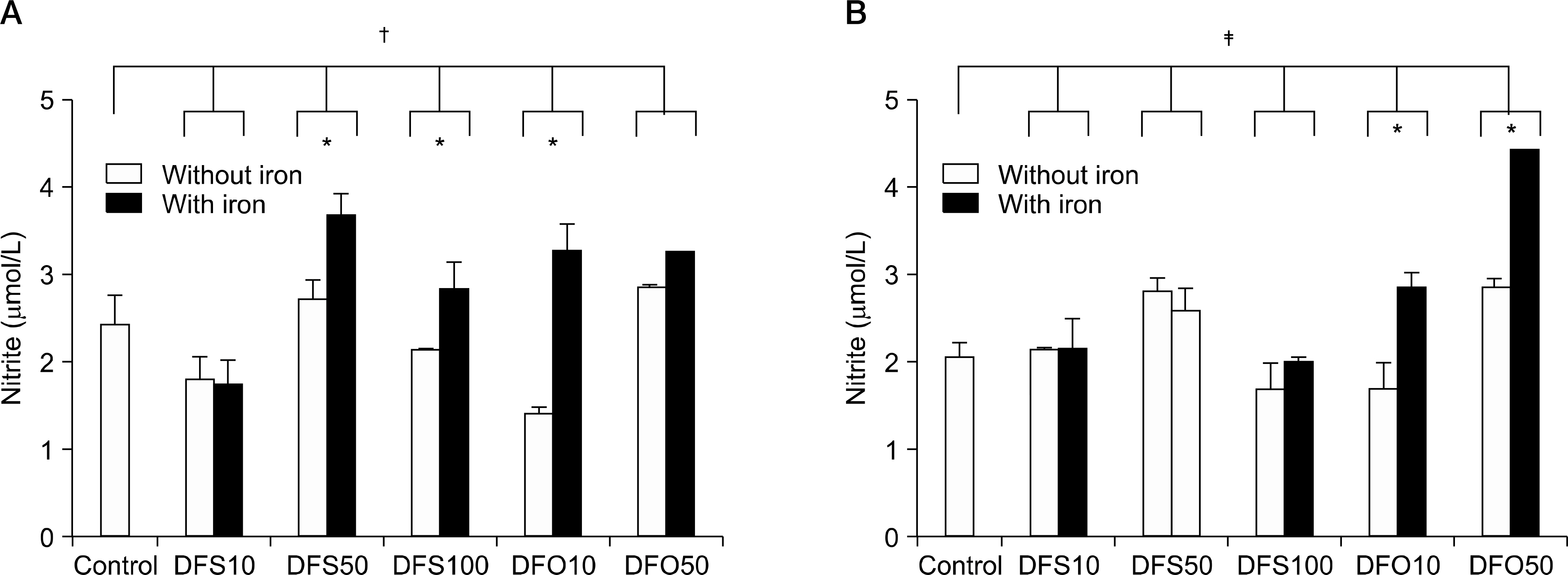
The viability of ICA was reported to be over 100%. Both DFS and DFO inhibited cell proliferation in a manner comparable to CSA. Cell proliferation without iron was reduced at the concentration of 100micrometer of DFO. With iron treatment, the reduction of the stimulation index was dependent on DFO concentrations. DFS decreased the proliferation without reference to the concentrations. After stimulation of phytohemagglutinin, the nitrite concentrations increased with iron. With lipopolysaccharides, the nitrite levels were higher in DFO with iron than control, but similar in DFS regardless of iron treatment. The levels of interleukin-2 were not different. Interleukin-10 was more abundantly produced in 50micrometer of DFO compared to DFS. Transforming growth factor-beta was higher in DFS than DFO at the low concentration, but opposite at the high concentration.
CONCLUSION
These data suggested that both iron chelating agents possessed immune suppressive effects comparable to CSA. The immunosuppressive effect of DFS may be distinct from DFO. More experiments are required to determine the exact mechanism of the immunosuppressive effect of DFS.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effects of oral iron chelator deferasirox on human malignant lymphoma cells
Jong Gwon Choi, Jung-Lim Kim, Joohee Park, Soonwook Lee, Seh Jong Park, Jun Suk Kim, Chul Won Choi
Korean J Hematol. 2012;47(3):194-201. doi: 10.5045/kjh.2012.47.3.194.
Reference
-
1). Heeney MM., Andrews NC. Iron homeostasis and inherited iron overload disorders: an overview. Hematol Oncol Clin N Am. 2004. 18:1379–403.
Article2). Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001. 131(Suppl):S616–S35.
Article3). Gabutti V., Borgna-Pignatti C. Clinical manifestations and therapy of transfusional haemosiderosis. Bail-lieres Clin Haematol. 1994. 7:919–40.4). Marx JJ. Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol. 2002. 15:411–26.
Article5). Cohen AR. New advances in iron chelation therapy. Hematology Am Soc Hematol Educ Program. 2006. 42–7.
Article6). Polson RJ., Jenkins R., Lombard M, et al. Mechanism of inhibition of mononuclear activation by the iron-chelating agent deferrioxamine. Immunology. 1990. 71:176–81.7). Kontoghiorghes GJ., Eracleous E., Economides C., Kolnagou A. Advances in iron overload therapies. Prospects for effective use of deferiprone (L1), deferoxamine, the new experimental chelators ICL670, GT56-252, L1NA11 and their combinations. Curr Med Chem. 2005. 12:2663–81.8). Kontoghiorghes GJ., Pattichi K., Hadjigavriel M., Kolnagou A. Transfusional iron overload and chelation therapy with deferoxamine and deferiprone (L1). Transfus Sci. 2000. 23:211–23.
Article9). Hileti D., Panayiotidis P., Hoffbrand V. Iron chelators induce apoptosis in proliferative cells. Br J Haematol. 1995. 89:181–7.10). Kim BM., Choi JY., Kim YJ., Woo HD., Chung HW. Desferrioxamine (DFX) has genotoxic effects on cultured human lymphocytes and induces the p53-mediated damage response. Toxicology. 2007. 229:226–35.
Article11). Soyano A., Chinea M., Romano EL. The effect of des-ferrioxamine on the proliferative response of rat lymphocytes stimulated with various mitogens in vitro. Immunopharmacology. 1984. 8:163–9.
Article12). Bowern N., Ramshaw IA., Badenoch-Jones P., Doherty PC. Effect of an iron-chelating agent on lymphocyte proliferation. Aust J Exp Biol Med Sci. 1984. 62:743–54.
Article13). Whitley WD., Hancock WW., Kupiec-Weglinishi JW., DeSousa M., Tilney NL. Iron chelation suppress mononuclear cell activation, modifies lymphocyte migration patterns, and prolongs rat cardiac allograft survival in rats. Transplantation. 1993. 56:1182–8.14). Hileti D., Panayiotidis P., Hoffbrand AV. Iron chelators induce apoptosis in proliferating cells. Br J Haematol. 1995. 89:181–7.
Article15). Cory JG., Lasater L., Sato A. Effect of iron-chelating agents on inhibitors of ribunucleotide reductase. Biochem Pharmacol. 1981. 30:979–84.16). Carutenuto P., Pontesilli O., Cambier JC., Hayward AR. Desferoximine blocks IL-2 receptor expression on human T lymphocytes. J Immunol. 1986. 136:2342–7.17). Crichton RR., Wilmet S., Legssyer R., Ward RJ. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J Inorganic Bio-chem. 2002. 91:9–18.
Article18). Emerit J., Beaumont C., Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmac-other. 2001. 55:333–9.
Article19). Fritsche G., Larcher C., Schennach H., Weiss G. Regulatory interactions between iron and nitric oxide metabolism for immune defense against Plasmo-diuim falciparum infection. J Infect Dis. 2001. 183:1388–94.20). Legssyer R., Josse C., Piette J., Ward RJ., Crichton RR. Changes in function of iron-loaded alvelolar marcro-phages after in vivo administration of desferriox-amine and/or chloroquine. J Inorganic Biochem. 2003. 94:36–42.21). Golenser J., Domb A., Mordechai-Daniel T., Leshem B., Luty A., Kremsner P. Iron chelators: correlation between effects on Plasmodium spp. and immune functions. J Parasitol. 2006. 92:170–7.
Article22). Yoon G., Kim HJ., Yoon YS., Cho H., Lim IK., Lee JH. Iron chelation-induced senescence-like growth arrest in hepatocyte cell lines: association of transforming growth factor beta1 (TGF-beta1)-mediated p27Kip1 expression. Biochem J. 2002. 366:613–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Tubulointerstitial Nephritis Induced by Deferasirox following Hematopoietic Stem Cell Transplantation for Severe Aplastic Anemia
- Economic Evaluation of Iron Chelation Agents: Oral Deferasirox versus Infusional Deferoxamine
- Effects of oral iron chelator deferasirox on human malignant lymphoma cells
- ReVersible Change of Vision in Patients Receiving Deferoxamine
- SARS-CoV-2 vaccine-elicited immune responses in solid organ transplant recipients