Korean J Hematol.
2008 Dec;43(4):258-262. 10.5045/kjh.2008.43.4.258.
Acute Tubulointerstitial Nephritis Induced by Deferasirox following Hematopoietic Stem Cell Transplantation for Severe Aplastic Anemia
- Affiliations
-
- 1Department of Pediatrics, Hanyang University Hospital, Hanyang University College of Medicine, Seoul, Korea. cord@hanyang.ac.kr
- KMID: 1485085
- DOI: http://doi.org/10.5045/kjh.2008.43.4.258
Abstract
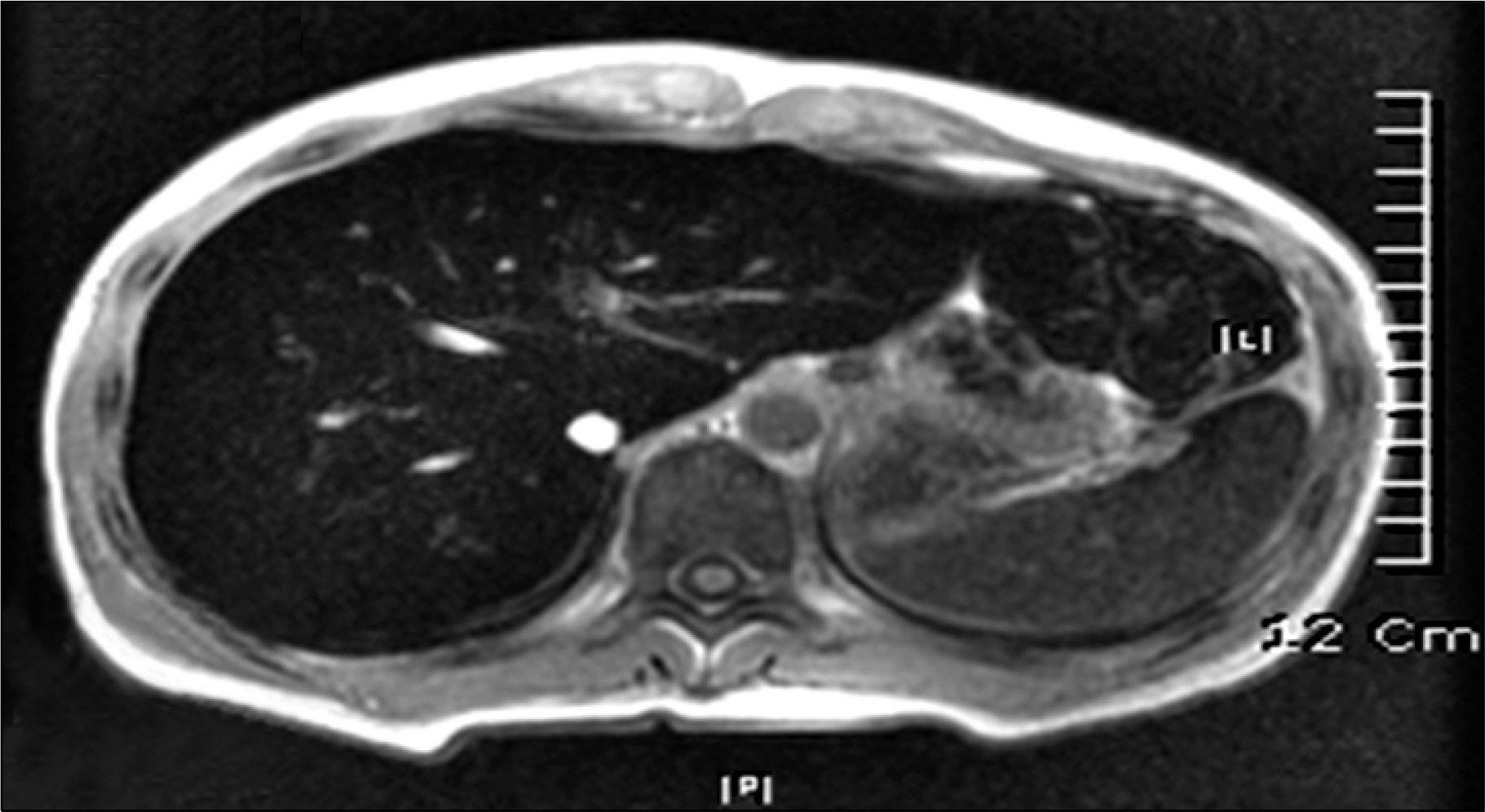
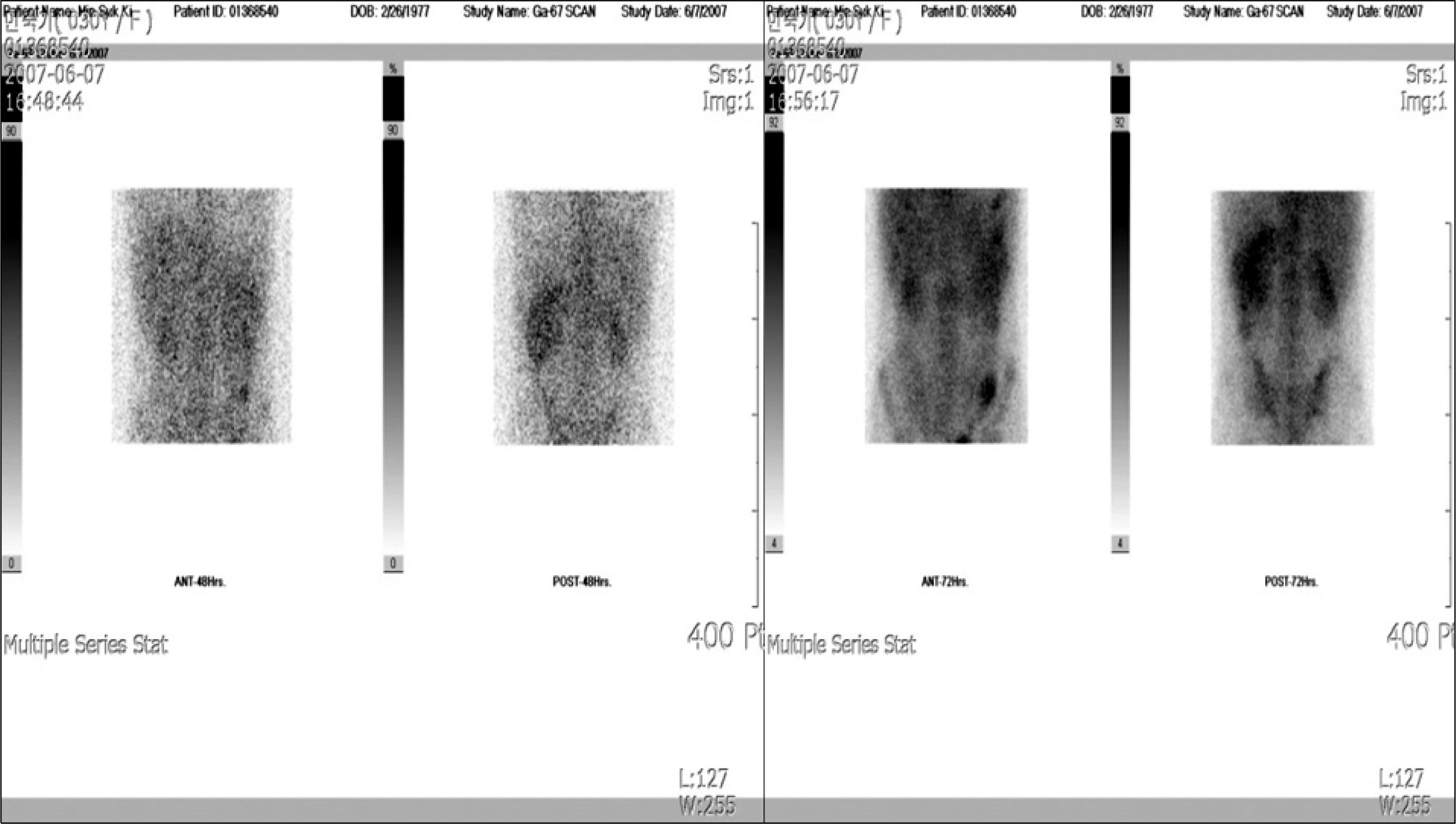
- Deferasirox is a once-daily, oral iron-chelating agent that is now widely available for the treatment of transfusional hemosiderosis. Deferasirox represents a significant advance in the treatment of iron overload, as the availability of an effective oral therapy has the potential to relieve many patients from the burden of frequent parenteral therapy with the previous reference standard iron chelator, deferoxamine. The well-known drug-related adverse events associated with deferasirox include gastrointestinal disturbances, rash, elevations in liver enzyme levels, and mild increases in serum creatinine levels, but acute renal failure is not common. The authors report a case of acute tubulointerstitial nephritis induced by deferasirox following hematopoietic stem cell transplantation for severe aplastic anemia
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinical and hematologic manifestations in patients with Diamond Blackfan anemia in Korea
Soon-Ki Kim, Hyo-Seop Ahn, Hee-Jo Back, Bin Cho, Eun-Jin Choi, Nak-Gyun Chung, Pyoung-Han Hwang, Dae-Chul Jeoung, Hyung-Jin Kang, Hyery Kim, Kyung-Nam Ko, Hong-Hoe Koo, Hoon Kook, Kwang-Chul Lee, Ho-Joon Lim, Young-Tak Lim, Chuhl-Joo Lyu, Jun-Eun Park, Kyung-Duk Park, Sang-Kyu Park, Kyung-Ha Ryu, Jong-Jin Seo, Hee-Young Shin, Ki-Woong Sung, Eun Sun Yoo
Korean J Hematol. 2012;47(2):131-135. doi: 10.5045/kjh.2012.47.2.131.
Reference
-
1). Ladis V., Chouliaras G., Berdousi H., Kanavakis E., Kattamis C. Longitudinal study of survival and causes of death in patients with thalassemia major in Greece. Ann N Y Acad Sci. 2005. 1054:445–50.
Article2). Roberts DJ., Rees D., Howard J., Hyde C., Alderson P., Brunskill S. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassemia. Cochrane Database Syst Rev. 2005. 4:CD004450.3). Borgna-Pignatti C., Cappellini MD., De Stefano P, et al. Cardiac morbidity and mortality in deferoxamine - or deferiprone-treated patients with thalassemia major. Blood. 2006. 107:3733–7.4). Porter JB. Practical management of iron overload. Br J Haematol. 2001. 115:239–52.
Article5). Treadwell MJ., Law AW., Sung J, et al. Barriers to adherence of deferoxamine usage in sickle cell disease. Pediatr Blood Cancer. 2005. 44:500–7.
Article6). Piga A., Galanello R., Forni GL, et al. Randomized phase II of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006. 91:873–80.7). Cappellini MD., Cohen A., Piga A, et al. A phase 3 study of (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006. 107:3455–62.8). Galanello R., Piga A., Forni GL, et al. Phase II clinical evaluation of deferasirox, a once-daily oral chelating agent, in pediatric with beta-thalassemia major. Hae-matologica. 2006. 91:1343–51.9). Porter J., Vichinsky E., Rose C, et al. A phase II study with oral ICL670 (ExjadeⓇ), a once-daily oral iron chelator, in patients with various transfusion- dependent anemias and iron overload. Blood. 2004. 104:872a.10). Cappellini MD., Taher A. Long-term experience with deferasirox (ICL670), a once-daily oral iron chelator, in the treatment of transfusional iron overload. Expert Opin Pharmacother. 2008. 9:2391–402.
Article11). Kontoghiorghes GJ., Neocleous K., Kolnagou A. Benefits and risks of deferiprone in iron overload in thalassaemia and other conditions: comparison of epidemiological and therapeutic aspects with deferoxamine. Drug Saf. 2003. 26:553–84.12). Borgna-Pignatti C., Rugolotto S., De Stefano P, et al. Survival and disease complications in thalassemia major. Ann N Y Acad Sci. 1998. 850:227–31.
Article13). Vichinsky E., Onyekwere O., Porter J, et al. A randomized comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol. 2007. 136:501–8.14). Kontoghiorghes GJ. Deferasirox: uncertain future following renal failure fatalities, agranulocytosis and other toxicities. Expert Opin Drug Saf. 2007. 6:235–9.
Article15). Iijima K., Hamahira K., Tanaka R, et al. Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int. 2002. 61:1801–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Papillary Thyroid Cancer in a Child with Very Severe Aplastic Anemia
- Successful engraftment after infusion of multiple low doses of CD34+ cells from a poorly matched sibling donor in a patient with severe aplastic anemia
- Aplastic Anemia
- Successful Selective CD34+ Cell Infusion after Late Graft Failure of Hematopoietic Stem Cell Transplantation in Two Cases of Severe Aplastic Anemia
- Case of Donor Cell Leukemia after Allogenic Bone Marrow Transplantation for Severe Aplastic Anemia