Korean J Hematol.
2012 Mar;47(1):8-16. 10.5045/kjh.2012.47.1.8.
Treatment strategies for Hodgkin lymphoma recurring following autologous hematopoietic stem cell transplantation
- Affiliations
-
- 1Department of Internal Medicine, University of Washington School of Medicine, Seattle, WA, USA.
- 2Division of Medical Oncology, University of Washington, Fred Hutchinson Cancer Research Center, Seattle, WA, USA. agopal@u.washington.edu
- KMID: 2251966
- DOI: http://doi.org/10.5045/kjh.2012.47.1.8
Abstract
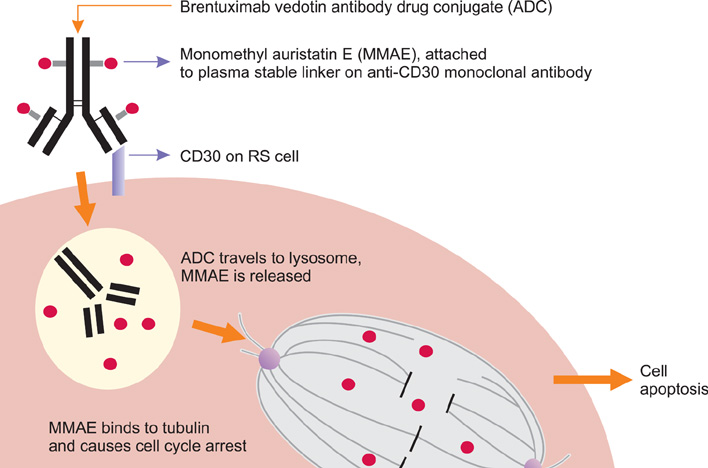
- Hodgkin lymphoma (HL) represents one of the great success stories in hematology going from a uniformly fatal disease, to one that is curable in the vast majority of cases. Despite this success, some patients experience relapse. To address this unmet need a variety of agents, classes of drugs, and strategies have demonstrated activity in HL recurring after autologous hematopoietic stem cell transplantation. These include chemotherapeutics (gemcitabine-based combinations, bendamustine), histone deacetylase (HDAC) inhibitors (panobinostat), immunomodulatory agents (lenalidomide), mTOR inhiobitors (everolimus), monoclonal antibodies (rituximab), and antibody-drug conjugates (brentuximab vedotin) as well the potential of long-term disease control via allogeneic transplantation. Such advances reflect our increased understanding of the biology of HL and hold promise for continued improved outcomes for those suffering with this condition.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010. 60:277–300.
Article2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN2008. Int J Cancer. 2010. 127:2893–2917.3. Viviani S, Bonadonna G, Santoro A, et al. Alternating versus hybrid MOPP and ABVD combinations in advanced Hodgkin's disease: ten-year results. J Clin Oncol. 1996. 14:1421–1430.
Article4. Armitage JO. Early-stage Hodgkin's lymphoma. N Engl J Med. 2010. 363:653–662.
Article5. Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002. 359:2065–2071.
Article6. Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001. 97:616–623.
Article7. Santoro A, Bredenfeld H, Devizzi L, et al. Gemcitabine in the treatment of refractory Hodgkin's disease: results of a multicenter phase II study. J Clin Oncol. 2000. 18:2615–2619.
Article8. Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007. 18:1071–1079.
Article9. Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin's disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003. 14:1762–1767.
Article10. Gopal AK, Press OW, Shustov AR, et al. Efficacy and safety of gemcitabine, carboplatin, dexamethasone, and rituximab in patients with relapsed/refractory lymphoma: a prospective multicenter phase II study by the Puget Sound Oncology Consortium. Leuk Lymphoma. 2010. 51:1523–1529.
Article11. Dennie TW, Kolesar JM. Bendamustine for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B-cell non-Hodgkin lymphoma. Clin Ther. 2009. 31:2290–2311.
Article12. De Filippi R, Aldinucci D, Galati D, et al. Effect of bendamustine on apoptosis and colony-initiating precursors in Hodgkin lymphoma cells. J Clin Oncol. 2011. 29:Suppl. abst e18559.
Article13. Moskowitz AJ, Hamlin PA, Gerecitano J, et al. Bendamustine is highly active in heavily pre-treated relapsed and refractory hodgkin lymphoma and serves as a bridge to allogeneic stem cell transplant. Blood. 2009. 114:abst 720.
Article14. Shao W, Growney J, Feng Y, et al. Potent anticancer activity of the pan-deacetylase inhibitor panobinostat (LBH589) as a single agent in in vitro and in vivo tumor models. AACR. 2008. abst 735.15. Dickinson M, Ritchie D, DeAngelo DJ, et al. Preliminary evidence of disease response to the pan deacetylase inhibitor panobinostat (LBH589) in refractory Hodgkin lymphoma. Br J Haematol. 2009. 147:97–101.
Article16. Sureda A, Younes A, Ben-Yehuda D, et al. Final analysis: phase II study of oral panobinostat in relapsed/refractory Hodgkin lymphoma patients following autologous hematopoietic stem cell transplant. Blood. 2010. 116:abst 419.
Article17. Kuruvilla J, Taylor D, Wang L, Blattler C, Keating A, Crump M. Phase II trial of lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2008. 112:abst 3052.
Article18. Fehniger TA, Larson S, Trinkaus K, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011. 118:5119–5125.
Article19. Boll B, Fuchs M, Reiners KS, Engert A, Borchmann P. Lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2010. 116:abst 2828.20. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008. 372:449–456.
Article21. Johnston PB, Inwards DJ, Colgan JP, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010. 85:320–324.
Article22. Rassidakis GZ, Medeiros LJ, Viviani S, et al. CD20 expression in Hodgkin and Reed-Sternberg cells of classical Hodgkin's disease: associations with presenting features and clinical outcome. J Clin Oncol. 2002. 20:1278–1287.
Article23. Younes A, Romaguera J, Hagemeister F, et al. A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer. 2003. 98:310–314.
Article24. Oki Y, Younes A. Does rituximab have a place in treating classic hodgkin lymphoma? Curr Hematol Malig Rep. 2010. 5:135–139.
Article25. Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J Clin Oncol. 2003. 21:3526–3534.
Article26. Katz J, Janik JE, Younes A. Brentuximab vedotin (SGN-35). Clin Cancer Res. 2011. 17:6428–6436.
Article27. Bartlett NL, Younes A, Carabasi MH, et al. Phase I study of SGN-30, a chimeric monoclonal antibody (mAb) in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2002. 100:362a–363a. (abst 1403).28. Bartlett NL, Younes A, Carabasi MH, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008. 111:1848–1854.
Article29. Forero-Torres A, Leonard JP, Younes A, et al. A phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009. 146:171–179.
Article30. Chen RW, Gopal AK, Smith SE, et al. Results from a pivotal phase II study of brentuximab vedotin (SGN-35) in patients with relapsed or refractory Hodgkin lymphoma (HL). J Clin Oncol. 2011. 29:Suppl. abst 8031.
Article31. Younes A, Barlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010. 363:1812–1821.
Article32. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007. 25:579–586.
Article33. Forero-Torres A, Berryman RB, Advani RH, et al. Prolonged treatment with brentuximab vedotin (SGN-35) in patients with relapsed or refractory Hodgkin lymphoma (HL) or systemic anaplastic large cell lymphoma (sALCL). Blood. 2011. 118:abst 3711.
Article34. Younes A, Connors JM, Park SI, Hunder NN, Ansell SM. Frontline therapy with brentuximab vedotin combined with ABVD or AVD in patients with newly diagnosed advanced stage Hodgkin lymphoma. Blood. 2011. 118:abst 955.
Article35. Chen RW, Forman SJ, Palmer J, et al. Brentuximab vedotin (SGN-35) enables successful reduced intensity allogeneic hematopoietic cell transplantation in relapsed/refractory Hodgkin lymphoma. Blood. 2011. 118:abst 664.
Article36. Burroughs LM, O'Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008. 14:1279–1287.
Article37. Tsai T, Goodman S, Saez R, et al. Allogeneic bone marrow transplantation in patients who relapse after autologous transplantation. Bone Marrow Transplant. 1997. 20:859–863.
Article38. Radich JP, Gooley T, Sanders JE, Anasetti C, Chauncey T, Appelbaum FR. Second allogeneic transplantation after failure of first autologous transplantation. Biol Blood Marrow Transplant. 2000. 6:272–279.
Article39. Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008. 26:455–462.
Article40. Anderlini P, Saliba R, Acholonu S, et al. Reduced-intensity allogeneic stem cell transplantation in relapsed and refractory Hodgkin's disease: low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant. 2005. 35:943–951.
Article41. Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin's lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008. 93:257–264.
Article42. Robinson SP, Sureda A, Canals C, et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin's lymphoma: identification of prognostic factors predicting outcome. Haematologica. 2009. 94:230–238.
Article43. Ram R, Gooley TA, Maloney DG, et al. Histology and time to progression predict survival for lymphoma recurring after reduced-intensity conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011. 17:1537–1545.
Article44. Gopal AK, Ramchandren R, Berryman RB, et al. Brentuximab vedotin (SGN-35) treatment in relapsed CD30-positive Hodgkin's lymphoma patients following allogeneic stem cell transplant: a multi-centre case series. EBMT. 2011. abst 0267.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autoimmune Diseases after Autologous Hematopoietic Stem Cell Transplantation in Patients with Non-Hodgkin's Lymphoma
- Two Cases of Hodgkin's Lymphoma after Allogeneic Hematopoietic Cell Transplantation
- Hodgkin's Lymphoma after Autologous Hematopoietic Stem Cell Transplantation for Angioimmunoblastic T-cell Lymphoma
- A Case of Mantle Cell Lymphoma Treated with Autologous Stem Cell Transplantation and Rituximab
- Treatment of Relapsed Hodgkin Lymphoma