Korean Circ J.
2008 May;38(5):244-249. 10.4070/kcj.2008.38.5.244.
Macrophage Depletion by Clodronate Liposomes Suppresses Neointimal Formation After Carotid Artery Injury in Apolipoprotein E-Deficient Mice
- Affiliations
-
- 1Department of Cardiology, Cardiovascular Center, Korea University Anam Hospital, Seoul, Korea. psyche94@hanmail.net
- 2Department of Cardiology, Gachon University Hospital, Incheon, Korea.
- 3Department of Cardiology, Korea University Guro Hospital, Seoul, Korea.
- KMID: 2225785
- DOI: http://doi.org/10.4070/kcj.2008.38.5.244
Abstract
-
BACKGROUND AND OBJECTIVES: Clodronate liposomes deplete phagocytic cells, thereby suppressing inflammation after vascular injury. We compared the effect of clodronate liposomes on macrophage depletion and neointimal formation in apolipoprotein E-deficient mice [ApoE (-) mice].
MATERIALS AND METHODS
ApoE (-) mice were randomly assigned to the clodronate liposomes group (Clodronate Group, n=7) and the vehicle liposomes group (Control Group, n=7). Clodronate (0.1 mL/10 g) was injected via the tail vein starting 2 days (d-2) before left common carotid artery injury.
RESULTS
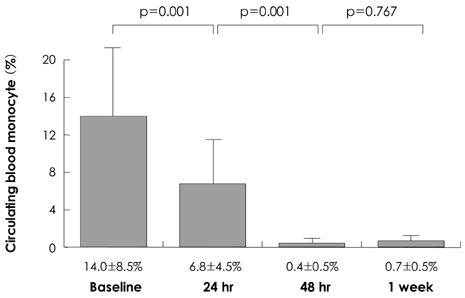
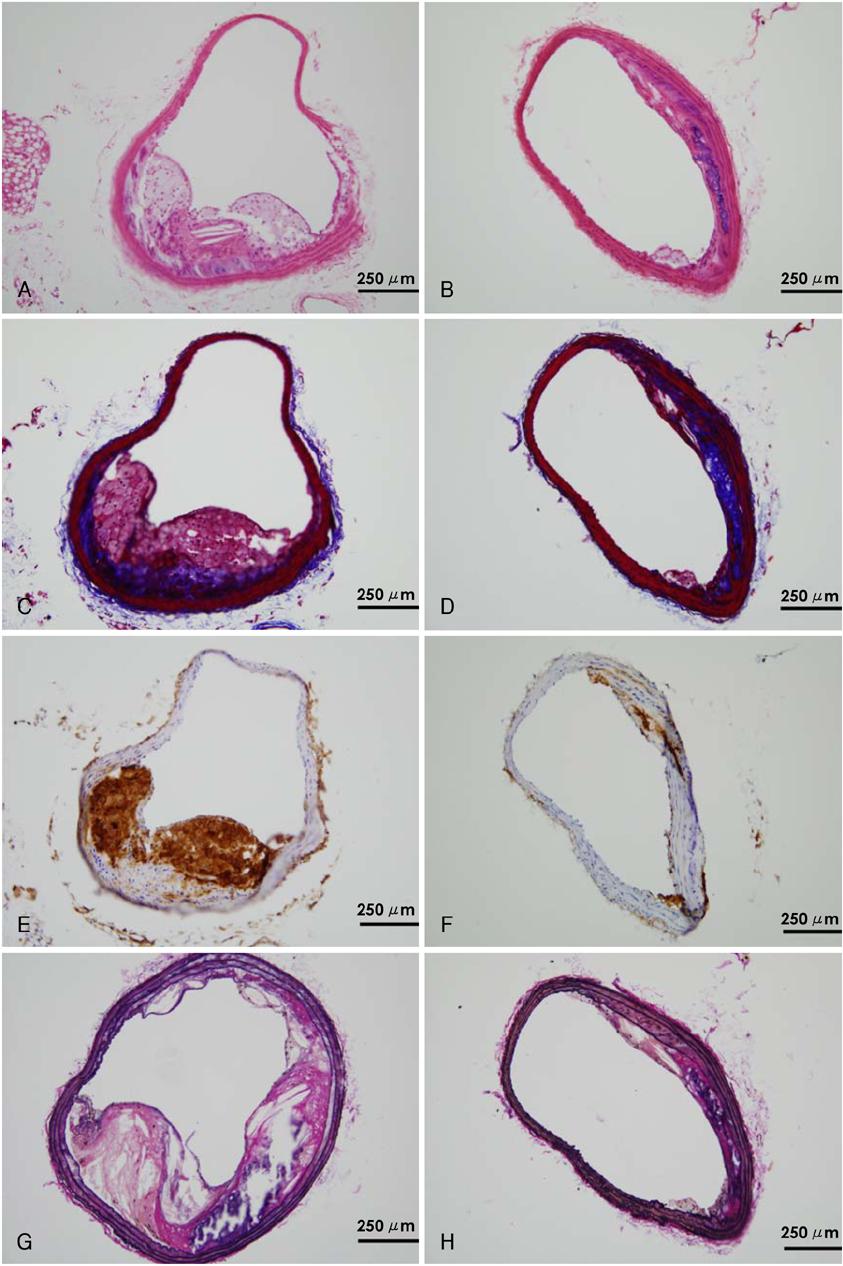
The percentage of blood monocytes was subsequently decreased after clodronate injection (14.0+/-7.4% at baseline, 6.8+/-4.9% at 24 hours and 0.7+/-0.3% at 1 week after the clodronate liposome injection). The percentage of macrophages in the plaque area was significantly lower in the clodronate group at week 2 (32.0+/-6.5 vs. 68.7+/-7.6%, respectively, p<0.05) and at week 4 (37.3+/-8.5 vs. 62.6+/-9.4%, respectively, p<0.05). The interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha concentrations were significantly decreased in the clodronate group at week 4 (12.3+/-2.5 vs. 22.9+/-3.5 pg/mL, respectively, p<0.05 for IL-6 and 16.6+/-2.2 vs. 43.6+/-6.1 pg/mL, respectively, p<0.05 for TNF-alpha). The plaque volume was significantly greater in the control group at week 2 (0.345+/-0.063 vs. 0.153+/-0.053 mm2, respectively, p<0.05) and at week 4 (0.320+/-0.027 vs. 0.167+/-0.070 mm2, respectively, p<0.05).
CONCLUSION
Intravenous administration of clodronate liposomes depleted monocytes and macrophages, and so this reduced the inflammatory markers and neointimal formation in ApoE (-) mice.
Keyword
MeSH Terms
-
Administration, Intravenous
Animals
Apolipoproteins
Apolipoproteins E
Carotid Arteries
Carotid Artery Injuries
Carotid Artery, Common
Clodronic Acid
Inflammation
Interleukin-6
Interleukins
Liposomes
Macrophages
Mice
Monocytes
Phagocytes
Tumor Necrosis Factor-alpha
Vascular System Injuries
Veins
Apolipoproteins
Apolipoproteins E
Clodronic Acid
Interleukin-6
Interleukins
Liposomes
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999. 340:115–126.2. Libby P. Inflammation in atherosclerosis. Nature. 2002. 420:868–874.3. van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994. 174:83–93.4. Wang YX. Cardiovascular functional phenotypes and pharmacological responses in apolipoprotein E deficient mice. Neurobiol Aging. 2005. 26:309–316.5. Schober A, Bernhagen J, Thiele M, et al. Stabilization of atherosclerotic plaques by blockade of macrophage migration inhibitory factor after vascular injury in apolipoprotein E-deficient mice. Circulation. 2004. 109:380–385.6. Rhee MY, Kim YK, Lee MY, Park BE, Kim DW, Cho MC. The effects of macrophage on neointimal formation after balloon or stent injury in hypercholesterolemic rabbits. Korean Circ J. 2005. 35:801–811.7. Matsumoto Y, Uwatoku T, Oi K, et al. Long-term inhibition of Rhokinase suppresses neointimal formation after stent implantation in porcine coronary arteries: involvement of multiple mechanisms. Arterioscler Thromb Vasc Biol. 2004. 24:181–186.8. Labarrere CA, Zaloga GP. C-reactive protein: from innocent bystander to pivotal mediator of atherosclerosis. Am J Med. 2004. 117:499–507.9. Wang YX, Halks-Miller M, Vergona R, et al. Increased aortic stiffness assessed by pulse wave velocity in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2000. 278:H428–H434.10. Schober A, Manka D, von Hundelshausen P, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002. 106:1523–1529.11. Kim DW, Kwon JS, Kim YG, et al. Novel oral formulation of paclitaxel inhibits neointimal hyperplasia in a rat carotid artery injury model. Circulation. 2004. 109:1558–1563.12. Farb A, John M, Acampado E, Kolodgie FD, Prescott MF, Virmani R. Oral everolimus inhibits in-stent neointimal growth. Circulation. 2002. 106:2379–2384.13. Lee JM, Moon KW, Yoo KD, et al. Inhibition of neointima formation by anti-vascular endothelial growth factor and receptor-1 peptides in a balloon-injured rat carotid artery. Korean Circ J. 2007. 37:475–482.14. Kang DH, Seung KB, Chang K, et al. Inhibition of neointimal hyperplasia by external radiation in rat carotid injury model: the possible role of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Korean Circ J. 1999. 29:944–955.15. Qian Q, Jutila MA, van Rooijen N, Cutler JE. Elimination of mouse splenic macrophages correlates with increased usceptibility to experimental disseminated candidiasis. J Immunol. 1994. 152:5000–5008.16. Hancock WW, Adams DH, Wyner LR, Sayegh MH, Karnovsky MJ. CD4+ mononuclear cells induce cytokine expression, vascular smooth muscle cell proliferation, and arterial occlusion after endothelial injury. Am J Pathol. 1994. 145:1008–1014.17. Mori E, Komori K, Yamaoka T, et al. Essential role of monocyte chemoattractant protein-1 in development of restenotic changes (neointimal hyperplasia and constrictive remodeling) after balloon angioplasty in hypercholesterolemic rabbits. Circulation. 2002. 105:2905–2910.18. Danenberg HD, Fishbein I, Gao J, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002. 106:599–605.19. Kwon JS, Park NK, Jeong IH, et al. A slight variation in the age of rats commonly used as a carotid artery injury model results in a large difference in neointima formation. Korean Circ J. 2007. 37:78–83.20. Wang L, Salu K, Verbeken E, et al. Stent-mediated methylprednisolone delivery reduces macrophage contents and in-stent neointimal formation. Coron Artery Dis. 2005. 16:237–243.21. Park SJ, Kim HS, Yang HM, et al. Thalidomide as a potent inhibitor of neointimal hyperplasia after balloon injury in rat carotid artery. Arterioscler Thromb Vasc Biol. 2004. 24:885–891.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Systemic macrophage depletion attenuates infarct size in an experimental mouse model of stroke
- The investigation of macrophage infiltration in the early phase of ischemic acute renal failure in mice
- The Effect of Estrogen Containing Liposome Local Delivery on the Neointimal Hyperplasia in the Rat Carotid Artery Balloon-Injury Model
- Intimal Hyperplasia in Loop-Injured Carotid Arteries Is Attenuated in Transglutaminase 2-Null Mice
- The neointimal hyperplasia effect of erythropoietin on carotid artery injury model of rat