J Cerebrovasc Endovasc Neurosurg.
2021 Dec;23(4):304-313. 10.7461/jcen.2021.E2021.04.003.
Systemic macrophage depletion attenuates infarct size in an experimental mouse model of stroke
- Affiliations
-
- 1Department of Neurosurgery, Chonnam National University Hospital and Medical School, Gwangju, Korea
- 2Department of Biomedical Sciences, Chonnam National University, Gwangju, Korea
- KMID: 2523888
- DOI: http://doi.org/10.7461/jcen.2021.E2021.04.003
Abstract
Objective
Macrophages have been shown to play important roles in various pathophysiological processes of the central nervous system via neuroinflammation, leading to an increased interest in macrophage biology. Circulating blood monocytes are among the first cells to infiltrate the brain after ischemic stroke; however, the role of innate immune cells such as monocytes and macrophages remains to be elucidated. Here, we investigated the association between blood monocytes and infarct size following ischemic stroke.
Methods
We induced stroke using a focal ischemia mouse model through middle cerebral artery suture occlusion. To deplete circulating blood monocytes, clodronate was injected intraperitoneally 24 h before the surgery. Animals were sacrificed at specified time points, and the infarct size and mRNA expression were then measured.
Results
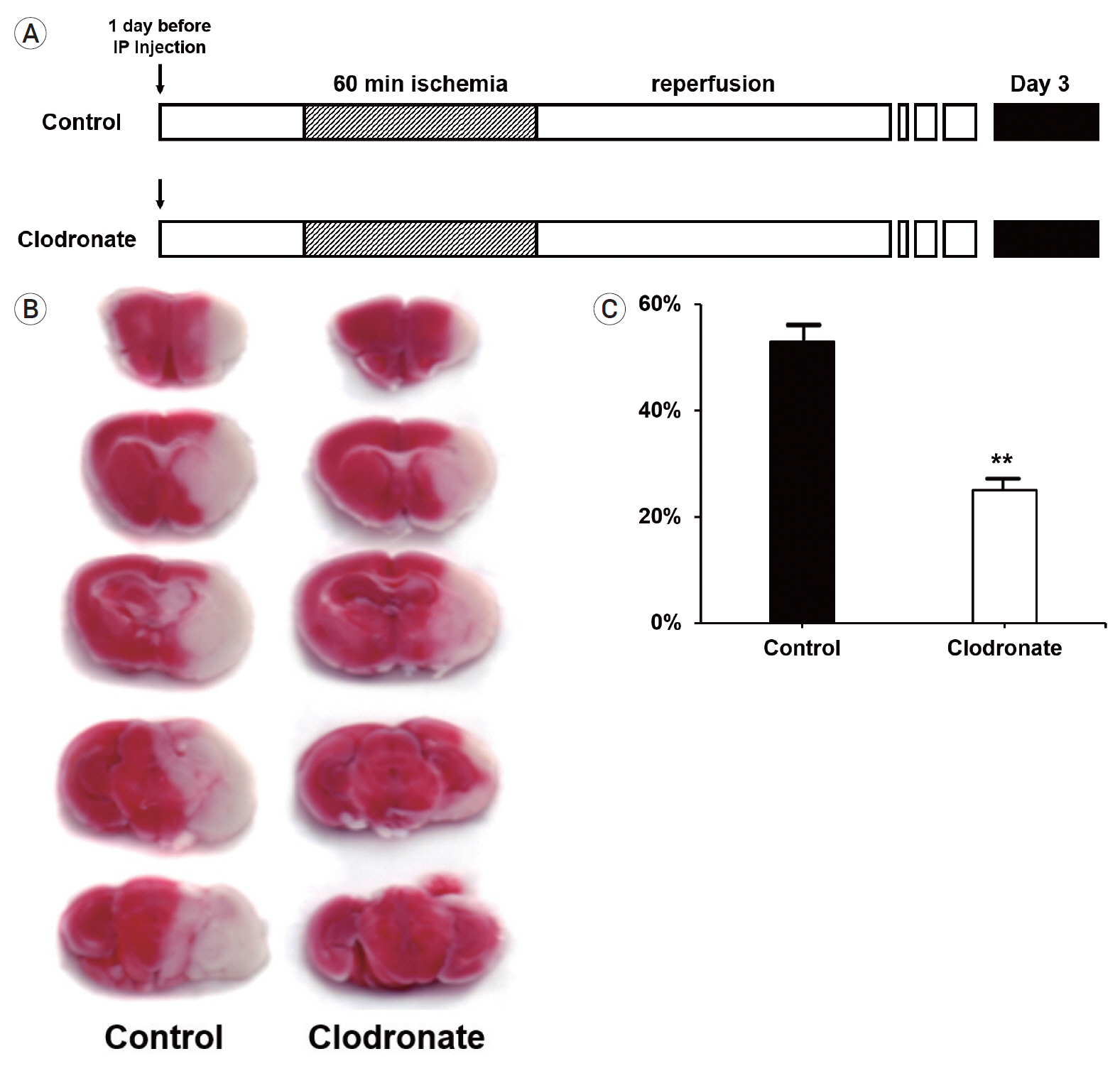
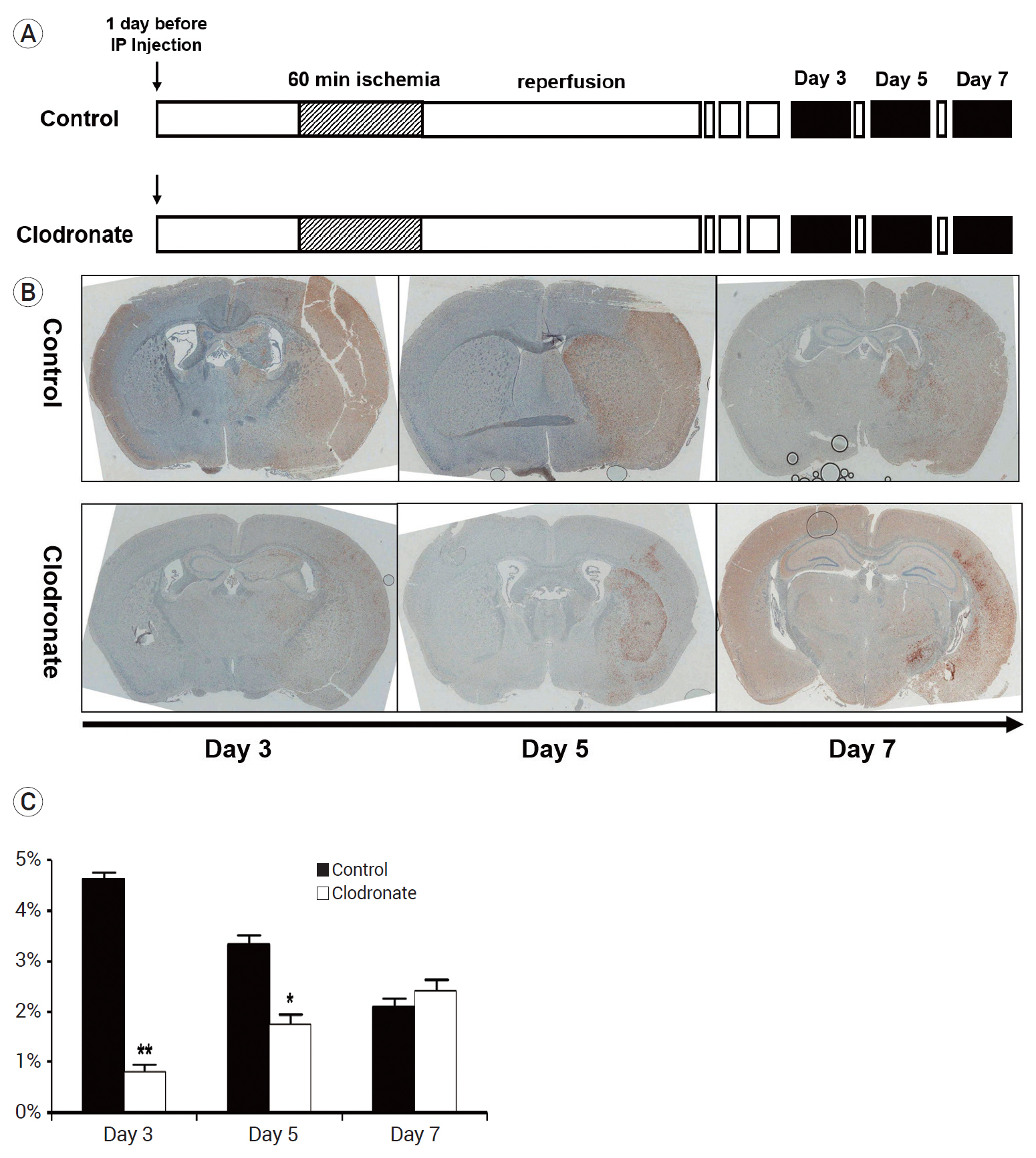
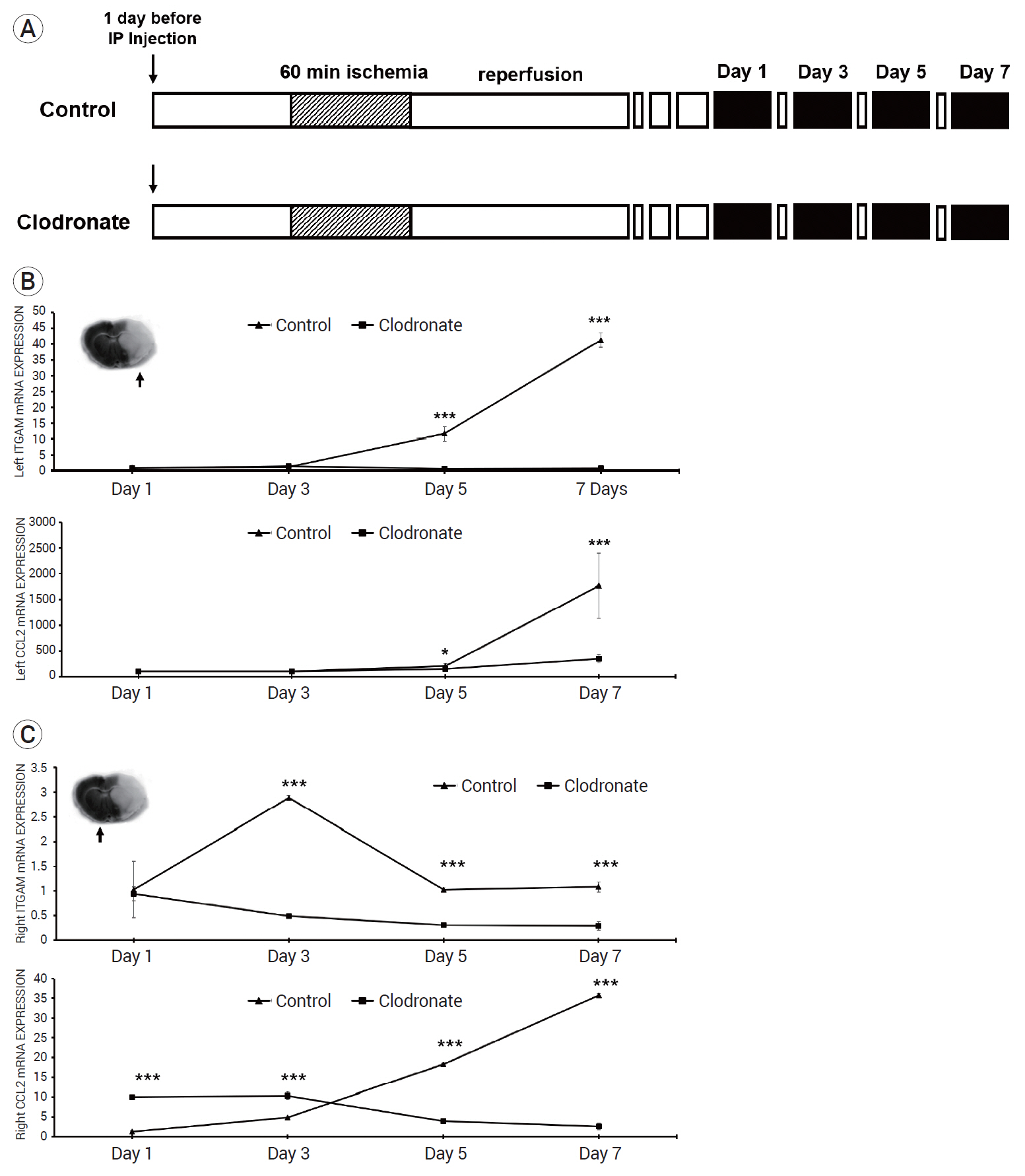
The clodronate-injected mice showed significantly smaller infarct size than the control mice. Immunohistochemical staining revealed that monocyte depletion significantly blocked the infiltration of macrophages and microglia. The mRNA expression levels of macrophage and microglia markers were higher in the left infarcted brain than in the right non-infarcted brain.
Conclusions
In summary, monocyte depletion reduced the infarct size and mitigated neurological deficits in mice following ischemic stroke, likely by blocking the infiltration of inflammatory cells such as macrophages and microglia.
Keyword
Figure
Reference
-
1. Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016; Mar. 113(12):E1738–46.
Article2. Chiba T, Umegaki K. Pivotal roles of monocytes/macrophages in stroke. Mediators Inflamm. 2013; 2013:759103.
Article3. Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007; May. 87(1):179–97.
Article4. Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009; May. 40(5):1849–57.
Article5. Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012; Jun. 71(6):743–52.
Article6. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015; Feb. 518(7540):547–51.
Article7. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003; Jan. 3(1):23–35.
Article8. Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000; Jan. 20(1):53–65.
Article9. Gunther A, Kuppers-Tiedt L, Schneider PM, Kunert I, Berrouschot J, Schneider D, et al. Reduced infarct volume and differential effects on glial cell activation after hyperbaric oxygen treatment in rat permanent focal cerebral ischaemia. Eur J Neurosci. 2005; Jun. 21(11):3189–94.10. Han X, Li Q, Lan X, El-Mufti L, Ren H, Wang J. Microglial depletion with clodronate liposomes increases proinflammatory cytokine levels, induces astrocyte activation, and damages blood vessel integrity. Mol Neurobiol. 2019; Sep. 56(9):6184–96.
Article11. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011; Jul. 17(7):796–808.
Article12. Jin X, Ishii H, Bai Z, Itokazu T, Yamashita T. Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One. 2012; 7(7):e41892.
Article13. Joo SP, Xie W, Xiong X, Xu B, Zhao H. Ischemic postconditioning protects against focal cerebral ischemia by inhibiting brain inflammation while attenuating peripheral lymphopenia in mice. Neuroscience. 2013; Jul. 243:149–57.
Article14. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009; Oct. 29(43):13435–44.
Article15. Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007; Mar. 27(10):2596–605.16. Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993; Jan. 7(1):19–24.
Article17. Lee JS, Song DJ, Hong JH, Kim TS, Joo SP. Diverse ischemic postconditioning protocols affect the infarction size in focal ischemic stroke. J Cerebrovasc Endovasc Neurosurg. 2018; Sep. 20(3):159–67.
Article18. Lehrmann E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, et al. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia. 1998; Dec. 24(4):437–48.19. Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000; Jul. 31(7):1735–43.
Article20. Makinde HM, Cuda CM, Just TB, Perlman HR, Schwulst SJ. Nonclassical monocytes mediate secondary injury, neurocognitive outcome, and neutrophil infiltration after traumatic brain injury. J Immunol. 2017; Nov. 199(10):3583–91.
Article21. Mirabelli-Badenier M, Braunersreuther V, Viviani GL, Dallegri F, Quercioli A, Veneselli E, et al. CC and CXC chemokines are pivotal mediators of cerebral injury in ischaemic stroke. Thromb Haemost. 2011; Mar. 105(3):409–20.
Article22. Na JI, Na JY, Choi WY, Lee MC, Park MS, Choi KH, et al. The HIF-1 inhibitor YC-1 decreases reactive astrocyte formation in a rodent ischemia model. Am J Transl Res. 2015; Apr. 7(4):751–60.23. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003; Jun. 23(6):748–55.24. Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011; Oct. 11(11):775–87.
Article25. Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003; Sep. 183(1):25–33.
Article26. Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005; Dec. 196(2):290–7.
Article27. Schmidt A, Strecker JK, Hucke S, Bruckmann NM, Herold M, Mack M, et al. Targeting different monocyte/macrophage subsets has no impact on outcome in experimental stroke. Stroke. 2017; Apr. 48(4):1061–9.
Article28. Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, et al. Migration of nhanced green fluorescent protein expressing bone marrow-derived microglia/ macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003; 117(3):531–9.29. Trahanas DM, Cuda CM, Perlman H, Schwulst SJ. Differential activation of infiltrating monocyte-derived cells after mild and severe traumatic brain injury. Shock. 2015; Mar. 43(3):255–60.
Article30. van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010; 605:189–203.
Article31. van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002; Feb-May. 12(1-2):81–94.
Article32. Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J Neurosci. 2016; Apr. 36(15):4182–95.
Article33. Xu X, Jiang Y. The Yin and Yang of innate immunity in stroke. Biomed Res Int. 2014; 2014:807978.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between Body Temperature and Infarct Size and Recovery in the Stroke
- Rat Models for Ischemic Stroke
- The Effects of UVB radiation on the immunologic function of mouse peritoneal macrophages
- Effects of Diphenylhydantoin on CBF, CMRO2, CMRG, and SEP in Experimental Cerebral Ischemia
- Photochemically Induced Cerebral Ischemia in a Mouse Model