Korean Circ J.
2008 Oct;38(10):507-513. 10.4070/kcj.2008.38.10.507.
Bio-Molecular Markers for Cardiovascular Disease: Significance of Natriuretic Peptides and Adrenomedullin
- Affiliations
-
- 1Division of Hypertension and Nephrology, Department of Medicine, National Cardiovascular Center, Osaka, Japan. thorio@ri.ncvc.go.jp
- KMID: 2225714
- DOI: http://doi.org/10.4070/kcj.2008.38.10.507
Abstract
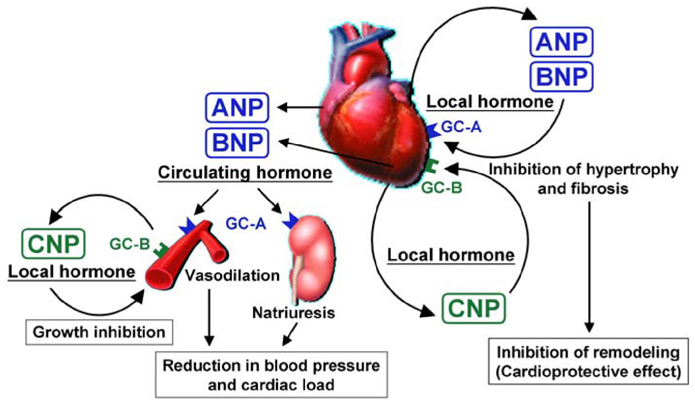
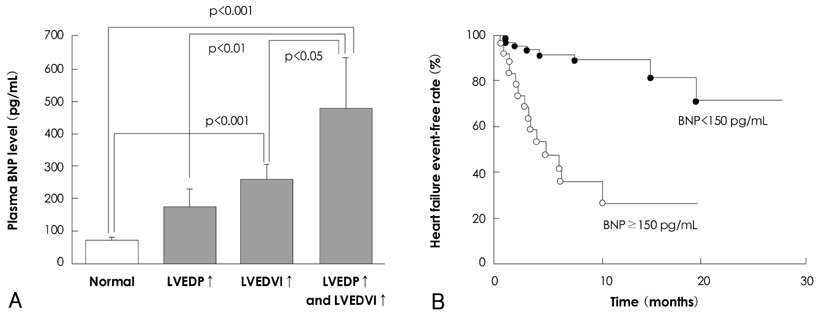
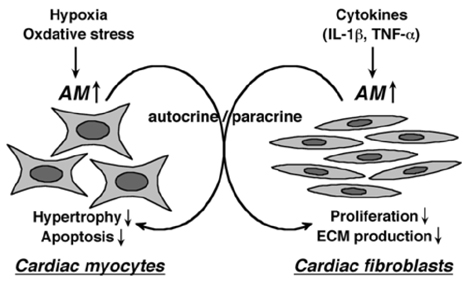
- There are many established and proposed bio-molecular markers for cardiovascular disease, including vasoactive substances, substances related to inflammation and oxidative stress, and substances involved in tissue structure and remodeling. Among these substances, we focused on natriuretic peptides and adrenomedullin (AM) as clinically useful bio-molecular markers in this review. Three natriuretic peptides-atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP)-play various important roles in the cardiovascular system. ANP and BNP are released from the heart and exist primarily as circulating hormones. They participate in the regulation of blood pressure and fluid levels. Plasma levels of ANP and BNP are increased in various pathological conditions such as heart failure, myocardial infarction, and hypertension with cardiac hypertrophy. BNP is now essential as a biochemical marker in managing patients with cardiovascular disease. CNP is mainly produced in vascular endothelium. It contributes to smooth muscle relaxation and growth inhibition as a local hormone, but it is also synthesized in cardiac fibroblasts and inhibits fibroblast proliferation and myocyte growth. However, the significance of plasma CNP levels remains to be elucidated. AM is widely distributed in various organs and tissues, including the cardiovascular system. Not only it is a potent vasodilator peptide, but it also has protective effects against vascular and cardiac cell injury and excessive growth. Plasma AM levels are increased in several cardiovascular diseases, including hypertension, heart failure, myocardial infarction, and atherosclerotic disease, and AM appears to be a predictive and prognostic marker in the setting of cardiovascular disease.
MeSH Terms
-
Adrenomedullin
Atherosclerosis
Atrial Natriuretic Factor
Biomarkers
Blood Pressure
Cardiomegaly
Cardiovascular Diseases
Cardiovascular System
Endothelium, Vascular
Fibroblasts
Heart
Heart Failure
Humans
Hypertension
Inflammation
Muscle Cells
Muscle, Smooth
Myocardial Infarction
Natriuretic Peptide, Brain
Natriuretic Peptide, C-Type
Natriuretic Peptides
Oxidative Stress
Plasma
Relaxation
Adrenomedullin
Atrial Natriuretic Factor
Natriuretic Peptide, Brain
Natriuretic Peptide, C-Type
Natriuretic Peptides
Figure
Cited by 1 articles
-
Preliminary Clinical Experience with Waon Therapy in Korea: Safety and Effect
Il-Suk Sohn, Jin-Man Cho, Woo-Shik Kim, Chong-Jin Kim, Kwon-Sam Kim, Jong-Hoa Bae, Chuwa Tei
J Cardiovasc Ultrasound. 2010;18(2):37-42. doi: 10.4250/jcu.2010.18.2.37.
Reference
-
1. Minamino N, Horio T, Nishikimi T. Kastin AJ, editor. Natriuretic peptides in the cardiovascular system. Handbook of Biologically Active Peptides. 2006. Burlington: Academic Press;1199–1207.2. Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993. 192:553–560.3. Yukimura T, Ito K, Takenaga T, Yamamoto K, Kangawa K, Matsuo H. Renal effects of a synthetic alpha-human atrial natriuretic polypeptide (alpha-hANP) in anesthetized dogs. Eur J Pharmacol. 1984. 103:363–366.4. Lang RE, Unger T, Ganten D. Atrial natriuretic peptide: a new factor in blood pressure control. J Hypertens. 1987. 5:255–271.5. Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension. 2000. 35:19–24.6. Maki T, Horio T, Yoshihara F, et al. Effect of neutral endopeptidase inhibitor on endogenous atrial natriuretic peptide as a paracrine factor in cultured cardiac fibroblasts. Br J Pharmacol. 2000. 131:1204–1210.7. Oliver PM, Fox JE, Kim R, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997. 94:14730–14735.8. Hayashi M, Tsutamoto T, Wada A, et al. Intravenous atrial natriuretic peptide prevents left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol. 2001. 37:1820–1826.9. Barr CS, Rhodes P, Struthers AD. C-type natriuretic peptide. Peptides. 1996. 17:1243–1251.10. Kalra PR, Anker SD, Struthers AD, Coats AJ. The role of C-type natriuretic peptide in cardiovascular medicine. Eur Heart J. 2001. 22:997–1007.11. Doi K, Ikeda T, Itoh H, et al. C-type natriuretic peptide induces redifferentiation of vascular smooth muscle cells with accelerated reendothelialization. Arterioscler Thromb Vasc Biol. 2001. 21:930–936.12. Horio T, Tokudome T, Maki T, et al. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003. 144:2279–2284.13. Tokudome T, Horio T, Soeki T, et al. Inhibitory effect of C-type natriuretic peptide (CNP) on cultured cardiac myocyte hypertrophy: interference between CNP and endothelin-1 signaling pathways. Endocrinology. 2004. 145:2131–2140.14. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994. 90:195–203.15. McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998. 351:9–13.16. Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction. JAMA. 2002. 288:1252–1259.17. Zoccali C, Mallamaci F, Benedetto FA, et al. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001. 12:1508–1515.18. Takami Y, Horio T, Iwashima Y, et al. Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am J Kidney Dis. 2004. 44:420–428.19. Nagaya N, Nishikimi T, Okano Y, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 1998. 31:202–208.20. Morita E, Yasue H, Yoshimura M, et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation. 1993. 88:82–91.21. Nishikimi T, Yoshihara F, Morimoto A, et al. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Hypertension. 1996. 28:22–30.22. Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000. 355:1126–1130.23. Kalra PR, Clague JR, Bolger AP, et al. Myocardial production of C-type natriuretic peptide in chronic heart failure. Circulation. 2003. 107:571–573.24. Ishiyama Y, Kitamura K, Ichiki Y, et al. Hemodynamic effects of a novel hypotensive peptide, human adrenomedullin, in rats. Eur J Pharmacol. 1993. 241:271–273.25. Horio T, Yoshihara F. Nishikimi T, editor. Biological action of adrenomedullin. Adrenomedullin in Cardiovascular Disease. 2005. New York: Springer;83–104.26. Ebara T, Miura K, Okumura M, et al. Effect of adrenomedullin on renal hemodynamics and functions in dogs. Eur J Pharmacol. 1994. 263:69–73.27. Nagaya N, Nishikimi T, Horio T, et al. Cardiovascular and renal effects of adrenomedullin in rats with heart failure. Am J Physiol. 1999. 276:R213–R218.28. Horio T, Kohno M, Kano H, et al. Adrenomedullin as a novel antimigration factor of vascular smooth muscle cells. Circ Res. 1995. 77:660–664.29. Sata M, Kakoki M, Nagata D, et al. Adrenomedullin and nitric oxide inhibit human endothelial cell apoptosis via a cyclic GMP-independent mechanism. Hypertension. 2000. 36:83–88.30. Miyashita K, Itoh H, Sawada N, et al. Adrenomedullin provokes endothelial Akt activation and promotes vascular regeneration both in vitro and in vivo. FEBS Lett. 2003. 544:86–92.31. Nagaya N, Satoh T, Nishikimi T, et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000. 101:498–503.32. Tsuruda T, Kato J, Kitamura K, et al. Adrenomedullin: a possible autocrine or paracrine inhibitor of hypertrophy of cardiomyocytes. Hypertension. 1998. 31:505–510.33. Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Effects of adrenomedullin on cultured rat cardiac myocytes and fibroblasts. Eur J Pharmacol. 1999. 382:1–9.34. Horio T, Nishikimi T, Yoshihara F, et al. Production and secretion of adrenomedullin in cultured rat cardiac myocytes and nonmyocytes: stimulation by interleukin-1β and tumor necrosis factor-α. Endocrinology. 1998. 139:4576–4580.35. Tokudome T, Horio T, Yoshihara F, et al. Adrenomedullin inhibits doxorubicin-induced cultured rat cardiac myocyte apoptosis via a cAMP-dependent mechanism. Endocrinology. 2002. 143:3515–3521.36. Ishimitsu T, Nishikimi T, Saito Y, et al. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest. 1994. 94:2158–2161.37. Nishikimi T, Matsuoka H, Ishikawa K, et al. Antihypertensive therapy reduces increased plasma levels of adrenomedullin and brain natriuretic peptide concomitant with regression of left ventricular hypertrophy in a patient with malignant hypertension. Hypertens Res. 1996. 19:97–101.38. Nishikimi T, Saito Y, Kitamura K, et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995. 26:1424–1431.39. Pousset F, Masson F, Chavirovskaia O, et al. Plasma adrenomedullin, a new independent predictor of prognosis in patients with chronic heart failure. Eur Heart J. 2000. 21:1009–1014.40. Richards AM, Doughty R, Nicholls MG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction. J Am Coll Cardiol. 2001. 37:1781–1787.41. Yoshihara F, Horio T, Nakamura S, et al. Adrenomedullin reflects cardiac dysfunction, excessive blood volume, and inflammation in hemodialysis patients. Kidney Int. 2005. 68:1355–1363.42. Kobayashi K, Kitamura K, Hirayama N, et al. Increased plasma adrenomedullin in acute myocardial infarction. Am Heart J. 1996. 131:676–680.43. Nagaya N, Nishikimi T, Uematsu M, et al. Plasma adrenomedullin as an indicator of prognosis after acute myocardial infarction. Heart. 1999. 81:483–487.44. Suzuki Y, Horio T, Hayashi T, et al. Plasma adrenomedullin concentration is increased in patients with peripheral arterial occlusive disease associated with vascular inflammation. Regul Pept. 2004. 118:99–104.45. Suzuki Y, Horio T, Nonogi H, et al. Adrenomedullin as a sensitive marker for coronary and peripheral arterial complications in patients with atherosclerotic risks. Peptides. 2004. 25:1321–1326.46. Nishida H, Horio T, Suzuki Y, et al. Plasma adrenomedullin as an independent predictor of future cardiovascular events in high-risk patients: comparison with C-reactive protein and adiponectin. Peptides. 2008. 29:599–605.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Implication of B-type Natriuretic Peptide in the Elderly
- Overview of Roles for Non-cardiac Natriuretic Peptides: Roles in Neural, Endocrine and Immune Systmes
- Role of biomarkers in the heart failure clinic
- Circulating Biologically Active Adrenomedullin Predicts Organ Failure and Mortality in Sepsis
- Effect of atrial natriuretic peptide on the proliferation and activity of osteoblastic cells