J Korean Surg Soc.
2012 Jul;83(1):21-29. 10.4174/jkss.2012.83.1.21.
Characterization of biological responses of colorectal cancer cells to anticancer regimens
- Affiliations
-
- 1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jckim@amc.seoul.kr
- 2Laboratory of Cancer Biology and Genetics, Asan Institute for Life Sciences, Seoul, Korea.
- 3Graduate School of East-West Medical Science, Kyung Hee University, Global Campus, Yongin, Korea.
- 4Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Division of Medical Genetics, Korea Research Institute of Bioscience & Biotechnology, Daejeon, Korea.
- KMID: 2212275
- DOI: http://doi.org/10.4174/jkss.2012.83.1.21
Abstract
- PURPOSE
Identification of subgroups of patients who differ in their response to treatment could help to establish which of the best available chemotherapeutic options are best, based on biological activity. In metastatic colorectal cancer (CRC), novel molecular-targeted agents that act on pathways that regulate cell growth, the cell cycle, apoptosis, angiogenesis, and invasion are being developed. Here, we employed an in vitro chemosensitivity assay to evaluate the biological efficacy of conventional monotherapies and combination chemotherapy with targeted drugs.
METHODS
The chemosensitivities of 12 CRC cell lines to the established regimens FOLFOX (5-fluorouracil [5-FU] + leucovorin + oxaliplatin) and FOLFIRI (5-FU + leucovorin + irinotecan) and to therapy with these regimens in combination with the biologically targeted drugs bevacizumab or cetuximab were comparatively evaluated for their effects on apoptotic and autophagic cell death processes, angiogenesis, and invasion.
RESULTS
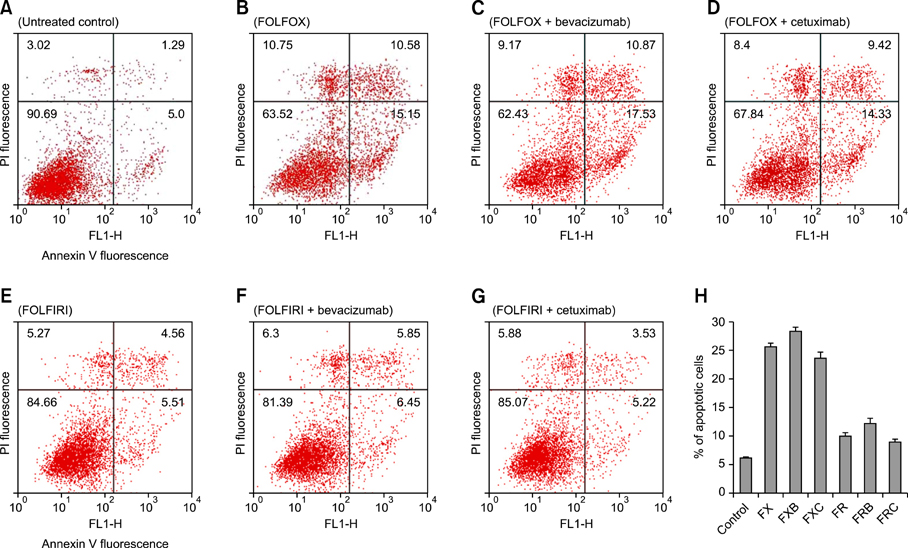
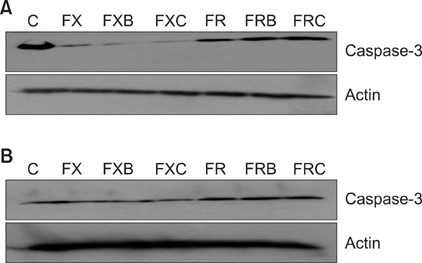
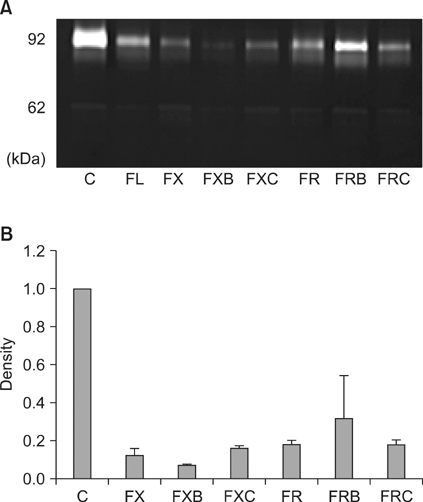
Each of the chemotherapeutic regimens promoted apoptotic cell death and invasion. All drug regimens caused significantly greater apoptotic cell death with activation of caspase-3 in SW480 cells compared to other cells, effects that were associated with a remarkable reduction in matrix metalloproteinase-9 activity. The FOLFOX regimen more effectively promoted apoptotic cell death, angiogenesis, and invasion than the FOLFIRI regimen. Combination therapy with FOLFOX/FOLFIRI regimen and bevacizumab produced a moderate angiogenesis-blocking effect in most cell lines.
CONCLUSION
The results validate our in vitro chemosensitivity assay, and suggest that it may be applied to help determine adequate regimens in individual CRC patients based on the biological characteristics of their tumors.
MeSH Terms
-
Antibodies, Monoclonal, Humanized
Antineoplastic Combined Chemotherapy Protocols
Apoptosis
Autophagy
Bevacizumab
Biomarkers, Pharmacological
Caspase 3
Cell Cycle
Cell Death
Cell Line
Cetuximab
Colorectal Neoplasms
Drug Therapy, Combination
Fluorouracil
Humans
Leucovorin
Matrix Metalloproteinase 9
Organoplatinum Compounds
Population Characteristics
Antibodies, Monoclonal, Humanized
Antineoplastic Combined Chemotherapy Protocols
Biomarkers, Pharmacological
Caspase 3
Fluorouracil
Leucovorin
Matrix Metalloproteinase 9
Organoplatinum Compounds
Figure
Reference
-
1. Kelly C, Cassidy J. Chemotherapy in metastatic colorectal cancer. Surg Oncol. 2007. 16:65–70.2. Sabharwal A, Kerr D. Chemotherapy for colorectal cancer in the metastatic and adjuvant setting: past, present and future. Expert Rev Anticancer Ther. 2007. 7:477–487.3. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004. 22:229–237.4. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000. 100:57–70.5. Ju JH, Chang SC, Wang HS, Yang SH, Jiang JK, Chen WC, et al. Changes in disease pattern and treatment outcome of colorectal cancer: a review of 5,474 cases in 20 years. Int J Colorectal Dis. 2007. 22:855–862.6. Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006. 130:2060–2073.7. Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, et al. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003. 200:183–194.8. Baker EA, Leaper DJ. Measuring gelatinase activity in colorectal cancer. Eur J Surg Oncol. 2002. 28:24–29.9. Baker EA, Leaper DJ. The plasminogen activator and matrix metalloproteinase systems in colorectal cancer: relationship to tumour pathology. Eur J Cancer. 2003. 39:981–988.10. Lubbe WJ, Zhou ZY, Fu W, Zuzga D, Schulz S, Fridman R, et al. Tumor epithelial cell matrix metalloproteinase 9 is a target for antimetastatic therapy in colorectal cancer. Clin Cancer Res. 2006. 12:1876–1882.11. Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004. 16:663–669.12. West NJ, Courtney ED, Poullis AP, Leicester RJ. Apoptosis in the colonic crypt, colorectal adenomata, and manipulation by chemoprevention. Cancer Epidemiol Biomarkers Prev. 2009. 18:1680–1687.13. Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005. 6:505–510.14. Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol. 2007. 78:217–245.15. Kim JC, Kim DD, Lee YM, Kim TW, Cho DH, Kim MB, et al. Evaluation of novel histone deacetylase inhibitors as therapeutic agents for colorectal adenocarcinomas compared to established regimens with the histoculture drug response assay. Int J Colorectal Dis. 2009. 24:209–218.16. Leman ES, Getzenberg RH. Nuclear structure as a source of cancer specific biomarkers. J Cell Biochem. 2008. 104:1988–1993.17. Nannizzi S, Veal GJ, Giovannetti E, Mey V, Ricciardi S, Ottley CJ, et al. Cellular and molecular mechanisms for the synergistic cytotoxicity elicited by oxaliplatin and pemetrexed in colon cancer cell lines. Cancer Chemother Pharmacol. 2010. 66:547–558.18. Kohne CH, Lenz HJ. Chemotherapy with targeted agents for the treatment of metastatic colorectal cancer. Oncologist. 2009. 14:478–488.19. Waldner MJ, Neurath MF. The molecular therapy of colorectal cancer. Mol Aspects Med. 2010. 31:171–178.20. Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005. 202:1691–1701.21. Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008. 118:2098–2110.22. Sun Q, Zheng Y, Liu Q, Cao X. Rapamycin reverses TLR4 signaling-triggered tumor apoptosis resistance by disrupting Akt-mediated Bcl-xL upregulation. Int Immunopharmacol. 2008. 8:1854–1858.23. Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010. 29:482–491.24. Lesterhuis WJ, de Vries IJ, Aarntzen EA, de Boer A, Scharenborg NM, van de Rakt M, et al. A pilot study on the immunogenicity of dendritic cell vaccination during adjuvant oxaliplatin/capecitabine chemotherapy in colon cancer patients. Br J Cancer. 2010. 103:1415–1421.25. Leibovitz A, Stinson JC, McCombs WB 3rd, McCoy CE, Mazur KC, Mabry ND. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976. 36:4562–4569.26. Huerta S, Heinzerling JH, Anguiano-Hernandez YM, Huerta-Yepez S, Lin J, Chen D, et al. Modification of gene products involved in resistance to apoptosis in metastatic colon cancer cells: roles of Fas, Apaf-1, NFkappaB, IAPs, Smac/DIABLO, and AIF. J Surg Res. 2007. 142:184–194.27. Rochette PJ, Bastien N, Lavoie J, Guerin SL, Drouin R. SW480, a p53 double-mutant cell line retains proficiency for some p53 functions. J Mol Biol. 2005. 352:44–57.28. Toscano F, Fajoui ZE, Gay F, Lalaoui N, Parmentier B, Chayvialle JA, et al. P53-mediated upregulation of DcR1 impairs oxaliplatin/TRAIL-induced synergistic anti-tumour potential in colon cancer cells. Oncogene. 2008. 27:4161–4171.29. Fujita H, Kato J, Horii J, Harada K, Hiraoka S, Shiraha H, et al. Decreased expression of hMLH1 correlates with reduced 5-fluorouracil-mediated apoptosis in colon cancer cells. Oncol Rep. 2007. 18:1129–1137.30. Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009. 360:563–572.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change of Natural Killer Cell in Patients with Colorectal Carcinoma
- Cell-based Immunotherapy for Colorectal Cancer with Cytokine-induced Killer Cells
- Silencing the livin gene enhances the cytotoxic effects of anticancer drugs on colon cancer cells
- Stimulatory Anticancer Effect of Resveratrol Mediated by G Protein-Coupled Estrogen Receptor in Colorectal Cancer
- Signal Transduction Pathways in Colorectal Cancer Carcinogenesis and Metastasis