J Korean Med Assoc.
2009 Oct;52(10):1007-1019. 10.5124/jkma.2009.52.10.1007.
Mitochondrial Permeability Transition Pore and Cardioprotection Against Ischemia-reperfusion Injury
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine/Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Korea. tren125@yuhs.ac
- KMID: 2188082
- DOI: http://doi.org/10.5124/jkma.2009.52.10.1007
Abstract
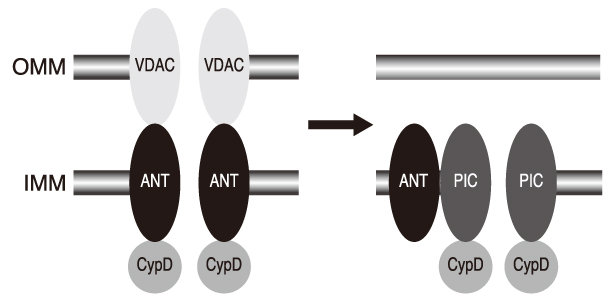
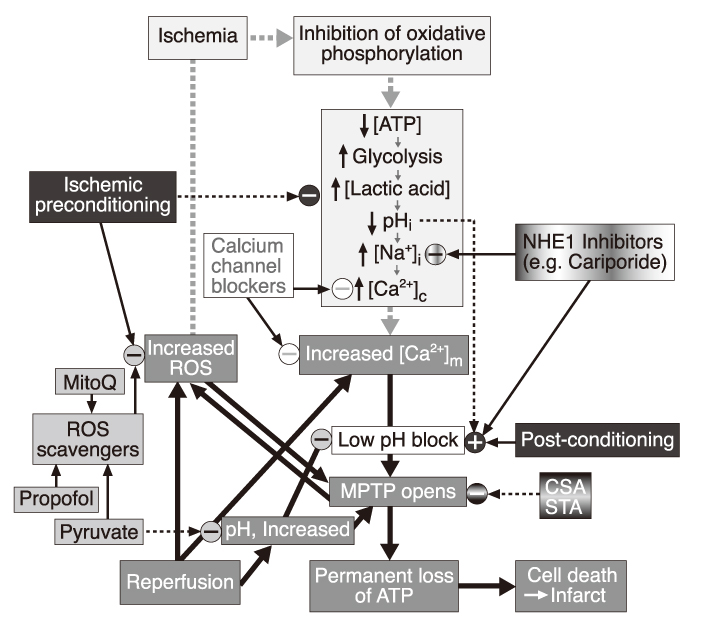
- Opening of mitochondrial permeability transition pore (mPTP) was found to have a critical role in cell death from ischemia/reperfusion (I/R) injury experimentally in the late 1980's. Thereafter, tremendous efforts have been made to define the molecular composition of mPTP and underlying mechanisms of its opening. mPTP opening, so far, has been demonstrated with the conformational changes of the mitochondrial protein components including cyclophilin-D, adenine nucleotide translocase, and voltage-dependent anion channel, which were induced by the modification of the levels of Ca2+, phosphate, mitochondrial membrane potential, intracellular pH and adenine nucleotide. At present, genetic modulation of the expression of protein components are being used in the investigation of its properties, presenting novel mechanisms of mPTP opening, including phosphate carrier. For therapeutic intervention, cyclosporin A and its analogues were first to be demonstrated to inhibit the opening of mPTP, affecting cyclophilin-D. There are numerous pharmacological substances that have direct or indirect effects on mPTP opening, including bongkrekic acid, reactive oxygen species scavengers, calcium channel blockers, and Na+/H+ exchanger-1 inhibitors, but only cyclosporin A was clinically tried to limit the myocardial infarction. Conditioning interventions, ischemic or anesthetic, have also been shown to be effective in limiting the detrimental effects of I/R injury. These interventions are commonly related to specific receptors on cell membrane and then signal transduction pathway consisting of many protein kinases, which eventually lead to mitochondria. And being presented are experimental evidences that inhibition of mPTP opening is a primary mechanism of these conditioning interventions. In conclusion, mPTP opening is now presented as primary mechanism and therapeutic target of I/R injury, but precise mechanism and standardized treatment method are needed to be clarified.
Keyword
MeSH Terms
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Adenine
Bongkrekic Acid
Calcium Channel Blockers
Cell Death
Cell Membrane
Cyclosporine
Hydrogen-Ion Concentration
Membrane Potential, Mitochondrial
Mitochondria
Mitochondrial ADP, ATP Translocases
Mitochondrial Membrane Transport Proteins
Mitochondrial Proteins
Myocardial Infarction
Myocardium
Permeability
Protein Kinases
Reactive Oxygen Species
Reperfusion Injury
Signal Transduction
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Adenine
Bongkrekic Acid
Calcium Channel Blockers
Cyclosporine
Mitochondrial ADP, ATP Translocases
Mitochondrial Membrane Transport Proteins
Mitochondrial Proteins
Protein Kinases
Reactive Oxygen Species
Figure
Reference
-
1. Crofts AR, Chappell JB. Calcium ion accumulation and volume changes of isolated liver mitochondria: reversal of calcium ion-induced swelling. Biochem J. 1965. 95:387–392.
Article2. Chappell JB, Crofts AR. Calcium ion accumulation and volume changes of isolated liver mitochondria: calcium ion-induced swelling. Biochem J. 1965. 95:378–386.
Article3. Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990. 258:755–786.
Article4. Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979. 195:460–467.5. Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979. 195:453–459.6. Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992. 267:2934–2939.
Article7. Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999. 341:233–249.
Article8. Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res. 2004. 61:372–385.
Article9. Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999. 79:1127–1155.
Article10. Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009. 46:850–857.
Article11. Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988. 255:357–360.12. Halestrap AP, Davidson AM. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990. 268:153–160.
Article13. Griffiths EJ, Halestrap AP. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. Biochem J. 1991. 274:611–614.
Article14. Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002. 277:34793–34799.
Article15. Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2002. 62:22–29.
Article16. Hansson MJ, Mattiasson G, Mansson R, Karlsson J, Keep MF, Waldmeier P, Ruegg UT, Dumont JM, Besseghir K, Elmer E. The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brainderived mitochondria. J Bioenerg Biomembr. 2004. 36:407–413.
Article17. Kay JE, Moore AL, Doe SE, Benzie CR, Schonbrunner R, Schmid FX, Halestrap AP. The mechanism of action of FK 506. Transplant Proc. 1990. 22:96–99.18. Basso E, Fante E, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mito-chondria devoid of Cyclophilin D. J Biol Chem. 2005. 280:18558–18561.
Article19. Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem. 1997. 272:3346–3354.
Article20. Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003. 10:1507–1525.
Article21. Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004. 427:461–465.
Article22. McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J. 2002. 367:541–548.
Article23. Szabo I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993. 330:206–210.24. Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol. 2001. 152:237–250.
Article25. Cesura AM, Pinard E, Schubenel R, Goetschy V, Friedlein A, Langen H, Polcic P, Forte MA, Bernardi P, Kemp JA. The voltage-dependent anion channel is the target for a new class of inhibitors of the mitochondrial permeability transition pore. J Biol Chem. 2003. 278:49812–49818.
Article26. Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem. 1998. 258:729–735.
Article27. Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007. 9:550–555.
Article28. Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem. 2008. 283:26312–26323.
Article29. Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J Biol Chem. 2008. 283:26307–26311.
Article30. Alcala S, Klee M, Fernandez J, Fleischer A, Pimentel-Muinos FX. A high-throughput screening for mammalian cell death effectors identifies the mitochondrial phosphate carrier as a regulator of cytochrome c release. Oncogene. 2008. 27:44–54.
Article31. McGuinness O, Yafei N, Costi A, Crompton M. The presence of two classes of high-affinity cyclosporin A binding sites in mitochondria. Evidence that the minor component is involved in the opening of an inner-membrane Ca2+-dependent pore. Eur J Biochem. 1990. 194:671–679.
Article32. Kim JS, Jin YJ, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH-and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol, Heart Circ Physiol. 2006. 290:2024–2034.33. Juhaszova M, Wang S, Zorov NH, Bradley DB, Gleichmann M, Mattson MP, et al. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann NY Acad Sci. 2008. 1123:197–212.
Article34. Halestrap AP. Calcium-dependent opening of a non-specific pore in the mitochondrial inner membrane is inhibited at pH values below 7-implications for the protective effect of low pH against chemical and hypoxic cell damage. Biochem J. 1991. 278:715–719.
Article35. Szabo I, Bernardi P, Zoratti M. Modulation of the mitochondrial megachannel by divalent cations and protons. J Biol Chem. 1992. 267:2940–2946.
Article36. Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003. 278:19062–19070.
Article37. Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007. 12:815–833.
Article38. Nicolli A, Petronilli V, Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry. 1993. 32:4461–4465.
Article39. Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976. 251:5069–5077.
Article40. Kristian T, Bernardi P, Siesjo BK. Acidosis promotes the permeability transition in energized mitochondria: implications for reperfusion injury. J Neurotrauma. 2001. 18:1059–1074.
Article41. Abramov AY, Fraley C, Diao CT, Winkfein R, Colicos MA, Duchen MR, French RJ, Pavlov E. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc Natl Acad Sci USA. 2007. 104:18091–18096.
Article42. Nicholls DG. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978. 176:463–474.
Article43. Bernardi P, Veronese P, Petronilli V. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. 1. Evidence for 2 separate Me2+ binding sites with opposing effects on the pore open probability. J Biol Chem. 1993. 268:1005–1010.
Article44. Halestrap AP. Mitochondria and reperfusion injury of the heart-a holey death but not beyond salvation. J Bioenerg Biomembr. 2009. 41:113–121.
Article45. Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004. 305:626–629.
Article46. Crompton M, Costi A. Kinetic evidence for a heart mitochondrial pore activated by Ca2+, inorganic phosphate and oxidative stress. A potential mechanism for mitochondrial dysfunction during cellular Ca2+ overload. Eur J Biochem. 1998. 178:489–501.
Article47. Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin-A. J Mol Cell Cardiol. 1991. 23:1351–1354.48. Leyssens A, Nowicky AV, Patterson L, Crompton M, Duchen MR. The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i, and [Ca2+]i studied in isolated rat cardiomyocytes. J Physiol. 1996. 496:111–128.
Article49. Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995. 307:93–98.
Article50. Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993. 25:1461–1469.
Article51. Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995. 307:93–98.
Article52. Javadov S, Karmazyn M, Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther. 2009. 06. 09. (Epub ahead of print).
Article53. Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003. 60:617–625.
Article54. Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Béjui F, Robert D, Ovize M. Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res. 2004. 61:115–122.55. Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007. 293:1654–1661.
Article56. Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007. 75:530–535.
Article57. Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003. 549:513–524.
Article58. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005. 434:658–662.
Article59. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005. 434:652–658.
Article60. Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005. 102:12005–12010.
Article61. Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005. 289:237–242.
Article62. Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André-Fouët X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008. 359:473–481.
Article63. Haworth RA, Hunter DR. Control of the mitochondrial permeability transition pore by high-affinity ADP binding at the ADP/ATP translocase in permeabilized mitochondria. J Bioenerg Biomembr. 2000. 32:91–96.64. Xu M, Wang Y, Hirai K, Ayub A, Ashraf M. Calcium preconditioning inhibits mitochondrial permeability transition and apoptosis. Am J Physiol Heart Circ Physiol. 2001. 280:899–908.
Article65. Gurevich RM, Regula KM, Kirshenbaum LA. Serpin protein CrmA suppresses hypoxia-mediated apoptosis of ventricular myocytes. Circulation. 2001. 103:1984–1991.
Article66. Rajesh KG, Sasaguri S, Suzuki R, Maeda H. Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. Am J Physiol Heart Circ Physiol. 2003. 285:2171–2178.
Article67. Kerr PM, Suleiman MS, Halestrap AP. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am J Physiol. 1999. 276:496–502.
Article68. Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005. 19:1088–1095.
Article69. de Jesús García-Rivas G, Guerrero-Hernández A, Guerrero-Serna G, Rodríguez-Zavala JS, Zazueta C. Inhibition of the mitochondrial calcium uniporter by the oxo-bridged dinuclear ruthenium amine complex (Ru360) prevents from irreversible injury in postischemic rat heart. FEBS J. 2005. 272:3477–3488.
Article70. Karmazyn M, Sostaric JV, Gan XT. The myocardial Na+/H+ exchanger: a potential therapeutic target for the prevention of myocardial ischaemic and reperfusion injury and attenuation of postinfarction heart failure. Drugs. 2001. 61:375–389.71. Javadov S, Choi A, Rajapurohitam V, Zeidan A, Basnakian AG, Karmazyn M. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res. 2008. 77:416–424.
Article72. Prendes MG, Torresín E, Gonzeález M, Fernández MA, Perazzo JC, Savino EA, Varela A. Protection of ischaemic-reperfused rat heart by dimethylamiloride is associated with inhibition of mitochondrial permeability transition. Clin Exp Pharmacol Physiol. 2008. 35:201–206.
Article73. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986. 74:1124–1136.
Article74. Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002. 55:534–543.
Article75. Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen . Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. J. Am J Physiol Heart Circ Physiol. 2003. 285:579–588.76. Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005. 111:194–197.
Article77. Warltier DC, al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988. 69:552–565.
Article78. Schlack W, Preckel B, Stunneck D, Thämer V. Effects of halothane, enflurane, isoflurane, sevoflurane and desflurane on myocardial reperfusion injury in the isolated rat heart. Br J Anaesth. 1998. 81:913–919.
Article79. Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005. 102:102–109.
Article80. Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anesthetic-induced Preconditioning Delays Opening of Mitochondrial Permeability Transition Pore via Protein Kinase Cε-mediated Pathway. Anesthesiology. 2009. 111:267.
Article81. Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3β. Anesthesiology. 2005. 103:987–995.
Article82. Bell SP, Sack MN, Patel A, Opie LH, Yellon DM. Delta opioid receptor stimulation mimics ischemic preconditioning in human heart muscle. J Am Coll Cardiol. 2000. 36:2296–2302.
Article83. Patel HH, Ludwig LM, Fryer RM, Hsu AK, Warltier DC, Gross GJ. Delta opioid agonists and volatile anesthetics facilitate cardioprotection via potentiation of KATP channel opening. FASEB J. 2002. 16:1468–1470.
Article84. Kokita N, Hara A, Abiko Y, Arakawa J, Hashizume H, Namiki A. Propofol improves functional and metabolic recovery in ischemic reperfused isolated rat hearts. Anesth Analg. 1998. 86:252–258.
Article85. Javadov SA, Lim KH, Kerr PM, Suleiman MS, Angelini GD, Halestrap AP. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res. 2000. 45:360–369.
Article86. Sztark F, Ichas F, Ouhabi R, Dabadie P, Mazat JP. Effects of the anaesthetic propofol on the calcium-induced permeability transition of rat heart mitochondria: direct pore inhibition and shift of the gating potential. FEBS Letter. 1995. 368:101–104.
Article87. Kawano T, Oshita S, Tsutsumi Y, Tomiyama Y, Kitahata H, Kuroda Y, Takahashi A, Nakaya Y. Clinically relevant concentrations of propofol have no effect on adenosine triphosphate-sensitive potassium channels in rat ventricular myocytes. Anesthesiology. 2002. 96:1472–1477.
Article88. Kato M, Akao M, Matsumoto-Ida M, Makiyama T, Iguchi M, Takeda T, Shimizu S, Kita T. The targeting of cyclophilin D by RNAi as a novel cardioprotective therapy: evidence from two-photon imaging. Cardiovasc Res. 2009. 83:335–344.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphine-induced postconditioning modulates mitochondrial permeability transition pore opening via delta-1 opioid receptors activation in isolated rat hearts
- Morphine and remifentanil-induced cardioprotection: its experimental and clinical outcomes
- Mechanism of Ischemia and Reperfusion Injury to the Heart: From the Viewpoint of Nitric Oxide and Mitochondria
- Cardioprotection and ageing
- Cardioprotective signaling cascade of A2 adenosine receptor agonist 5'-N-ethylcarboxaminidoadenosine against myocardial reperfusion injury