Endocrinol Metab.
2014 Sep;29(3):363-370. 10.3803/EnM.2014.29.3.363.
Vav3, a GEF for RhoA, Plays a Critical Role under High Glucose Conditions
- Affiliations
-
- 1Department of Anatomy, Korea University College of Medicine, Seoul, Korea. anatomykim@korea.ac.kr
- 2Bando Hospital, Seoul, Korea.
- 3Division of Cardiology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2169474
- DOI: http://doi.org/10.3803/EnM.2014.29.3.363
Abstract
- BACKGROUND
The role of small GTPase molecules is poorly understood under high glucose conditions.
METHODS
We analyzed the expression pattern of Vav3 in skeletal muscle C2C12 cells under high glucose culture condition with reverse transcription-polymerase chain reaction and Western blot analysis. We also measured glucose uptake using isotope-labelled glucose.
RESULTS
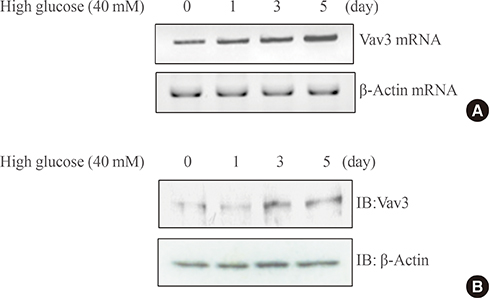
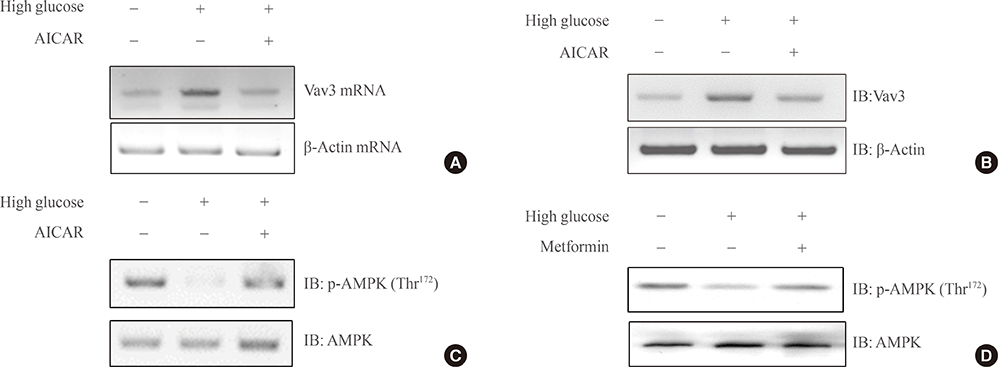
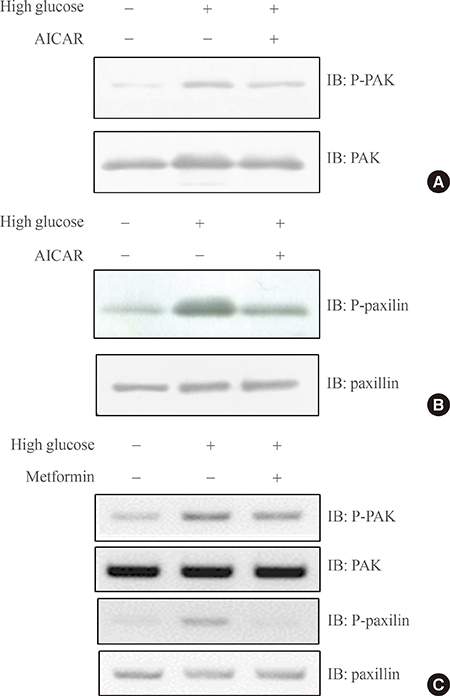
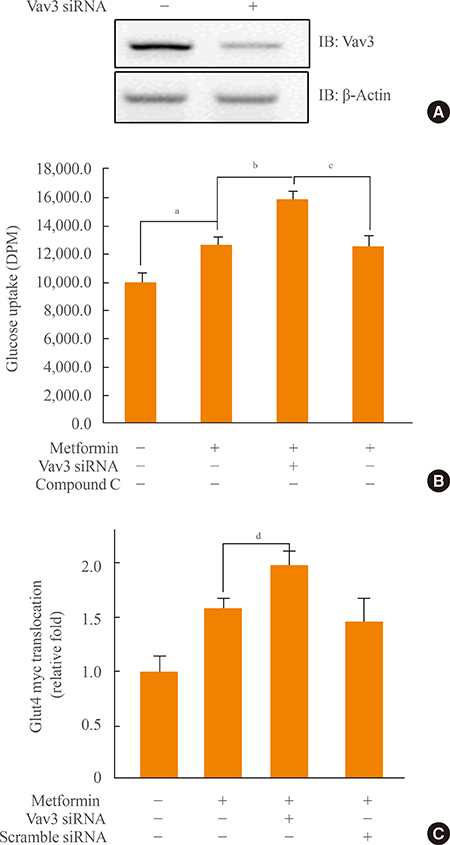
We showed that expression of Vav3 (a guanine nucleotide exchange factor for RhoA) increased. mRNA and protein levels in skeletal muscle C2C12 cells under high glucose conditions. The AMP-activated protein kinase (AMPK) activator AMPK agonist 5-aminoimidazole-4-carboxy-amide-1-d-ribofuranoside (AICAR) suppressed high glucose-induced Vav3 induction. In addition, exposure of cells to high glucose concentration increased the phosphorylation of PAK-1, a molecule downstream of RhoA. The phosphorylation of paxillin, a downstream molecule of PAK-1, was also increased by exposure to high glucose. Phosphorylation of these molecules was not observed in the presence of AICAR, indicating that AMPK is involved in the RhoA signal pathway under high glucose conditions. Knock down of Vav3 enhances metformin-mediated glucose uptake. Inhibition of AMPK blocked the increases of Vav3 knock down-induced glucose uptake. Metformin-mediated Glut4 translocation was also increased by Vav3 knock-down, suggesting that Vav3 is involved in metformin-mediated glucose uptake.
CONCLUSION
These results demonstrate that Vav3 is involved in the process of metformin-mediated glucose regulation.
Keyword
MeSH Terms
-
AMP-Activated Protein Kinases
Blotting, Western
Glucose*
GTP Phosphohydrolases
Guanine Nucleotide Exchange Factors
Metformin
Muscle, Skeletal
Paxillin
Phosphorylation
RNA, Messenger
Signal Transduction
AMP-Activated Protein Kinases
GTP Phosphohydrolases
Glucose
Guanine Nucleotide Exchange Factors
Metformin
Paxillin
RNA, Messenger
Figure
Reference
-
1. Siegel Gj, Agranoff BW, Albers RW, Fisher SK, Uhler MD. Chapter 31, Circulation and energy metabolism of the brain. Basic Neurochemistry. 6th ed. Philadelphia: Lippincott-Raven;1999. p. 637–670.2. Despres JP. Potential contribution of metformin to the management of cardiovascular disease risk in patients with abdominal obesity, the metabolic syndrome and type 2 diabetes. Diabetes Metab. 2003; 29(4 Pt 2):6S53–6S61.3. Bailey CJ, Day C. Avandamet: combined metformin-rosiglitazone treatment for insulin resistance in type 2 diabetes. Int J Clin Pract. 2004; 58:867–876.4. Bailey CJ. Metformin: effects on micro and macrovascular complications in type 2 diabetes. Cardiovasc Drugs Ther. 2008; 22:215–224.5. Kooy A, de Jager J, Lehert P, Bets D, Wulffele MG, Donker AJ, Stehouwer CD. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009; 169:616–625.6. Borst SE, Snellen HG. Metformin, but not exercise training, increases insulin responsiveness in skeletal muscle of Sprague-Dawley rats. Life Sci. 2001; 69:1497–1507.7. Teranishi T, Ohara T, Maeda K, Zenibayashi M, Kouyama K, Hirota Y, Kawamitsu H, Fujii M, Sugimura K, Kasuga M. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism. 2007; 56:1418–1424.8. He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009; 137:635–646.9. Yoshida T, Okuno A, Tanaka J, Takahashi K, Nakashima R, Kanda S, Ogawa J, Hagisawa Y, Fujiwara T. Metformin primarily decreases plasma glucose not by gluconeogenesis suppression but by activating glucose utilization in a non-obese type 2 diabetes Goto-Kakizaki rats. Eur J Pharmacol. 2009; 623:141–147.10. Wiernsperger NF. Membrane physiology as a basis for the cellular effects of metformin in insulin resistance and diabetes. Diabetes Metab. 1999; 25:110–127.11. Pryor PR, Liu SC, Clark AE, Yang J, Holman GD, Tosh D. Chronic insulin effects on insulin signalling and GLUT4 endocytosis are reversed by metformin. Biochem J. 2000; 348(Pt 1):83–91.12. Wang Y, Li XJ, Zhang M, Wu YH, Tong NW, Zhao TY, Li J. The effect of metformin on insulin receptor protein tyrosine kinase activity of HIT-T15 cell exposed to high concentration glucose and free fatty acid. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007; 38:819–821.13. Hardie DG, Carling D. The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur J Biochem. 1997; 246:259–273.14. Makinde AO, Gamble J, Lopaschuk GD. Upregulation of 5'-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ Res. 1997; 80:482–489.15. Ai H, Ihlemann J, Hellsten Y, Lauritzen HP, Hardie DG, Galbo H, Ploug T. Effect of fiber type and nutritional state on AICAR- and contraction-stimulated glucose transport in rat muscle. Am J Physiol Endocrinol Metab. 2002; 282:E1291–E1300.16. Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002; 99:15983–15987.17. Henin N, Vincent MF, Gruber HE, Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995; 9:541–546.18. Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002; 282:E18–E23.19. Ju JS, Gitcho MA, Casmaer CA, Patil PB, Han DG, Spencer SA, Fisher JS. Potentiation of insulin-stimulated glucose transport by the AMP-activated protein kinase. Am J Physiol Cell Physiol. 2007; 292:C564–C572.20. Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002; 51:2886–2894.21. Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004; 116:167–179.22. Ueda S, Kitazawa S, Ishida K, Nishikawa Y, Matsui M, Matsumoto H, Aoki T, Nozaki S, Takeda T, Tamori Y, Aiba A, Kahn CR, Kataoka T, Satoh T. Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. FASEB J. 2010; 24:2254–2261.23. JeBailey L, Rudich A, Huang X, Di Ciano-Oliveira C, Kapus A, Klip A. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol Endocrinol. 2004; 18:359–372.24. JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes. 2007; 56:394–403.25. Ueda S, Kataoka T, Satoh T. Activation of the small GTPase Rac1 by a specific guanine-nucleotide-exchange factor suffices to induce glucose uptake into skeletal-muscle cells. Biol Cell. 2008; 100:645–657.26. Moores SL, Selfors LM, Fredericks J, Breit T, Fujikawa K, Alt FW, Brugge JS, Swat W. Vav family proteins couple to diverse cell surface receptors. Mol Cell Biol. 2000; 20:6364–6373.27. Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000; 20:1461–1477.28. Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998; 279:558–560.29. Cichowski K, Brugge JS, Brass LF. Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95vav, in platelets. J Biol Chem. 1996; 271:7544–7550.30. Gotoh A, Takahira H, Geahlen RL, Broxmeyer HE. Cross-linking of integrins induces tyrosine phosphorylation of the proto-oncogene product Vav and the protein tyrosine kinase Syk in human factor-dependent myeloid cells. Cell Growth Differ. 1997; 8:721–729.31. Miranti CK, Leng L, Maschberger P, Brugge JS, Shattil SJ. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr Biol. 1998; 8:1289–1299.32. Zheng L, Sjolander A, Eckerdal J, Andersson T. Antibody-induced engagement of beta 2 integrins on adherent human neutrophils triggers activation of p21ras through tyrosine phosphorylation of the protooncogene product Vav. Proc Natl Acad Sci U S A. 1996; 93:8431–8436.33. Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989; 8:2283–2290.34. Adams JM, Houston H, Allen J, Lints T, Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene. 1992; 7:611–618.35. Katzav S, Cleveland JL, Heslop HE, Pulido D. Loss of the amino-terminal helix-loop-helix domain of the vav proto-oncogene activates its transforming potential. Mol Cell Biol. 1991; 11:1912–1920.36. Henske EP, Short MP, Jozwiak S, Bovey CM, Ramlakhan S, Haines JL, Kwiatkowski DJ. Identification of VAV2 on 9q34 and its exclusion as the tuberous sclerosis gene TSC1. Ann Hum Genet. 1995; 59(Pt 1):25–37.37. Schuebel KE, Bustelo XR, Nielsen DA, Song BJ, Barbacid M, Goldman D, Lee IJ. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 1996; 13:363–371.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of RhoA-mediated Chemoresistance in Gastric Cancer Cells
- Expression of RhoA in Colorectal Cancers and Its Clinicopathological Significance
- ADRB3, ROCK2, and GEF Levels in Overactive Bladder Patients
- p190RhoGAP and Rap-dependent RhoGAP (ARAP3) inactivate RhoA in response to nerve growth factor leading to neurite outgrowth from PC12 cells
- Discovery of Cellular RhoA Functions by the Integrated Application of Gene Set Enrichment Analysis