Cancer Res Treat.
2005 Aug;37(4):251-256.
Characterization of RhoA-mediated Chemoresistance in Gastric Cancer Cells
- Affiliations
-
- 1Cancer Center, Samsung Medical Center, and Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul, Korea. cpark@smc.samsung.co.kr
Abstract
- PURPOSE
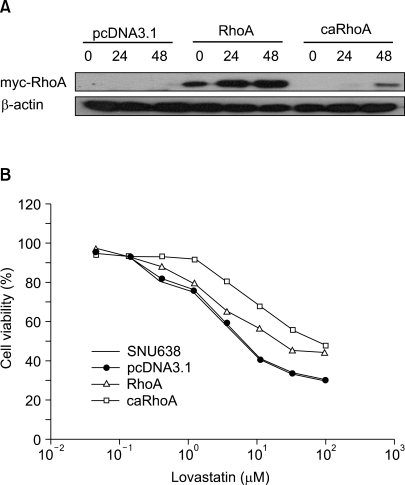
RhoA is a critical transducer of extracellular signals, which leads to organization of actin cytoskeleton, motility, adhesion and gene regulation. The present study aimed to explore whether RhoA influences the susceptibility of gastric cancer cells to chemotherapeutic drugs. MATERIALS AND METHODS: SNU638 cells were transfected with a mock vector (pcDNA3.1), RhoA (pcDNA/RhoA), or constitutively active RhoA (pcDNA/caRhoA). MTT assay and Western blot analysis were performed to study the growth response to several chemotherapeutic drugs in the gastric cancer cell line, SNU638, with different RhoA levels. RESULTS: RhoA significantly enhanced the resistance to lovastatin, 5-FU, taxol and vincristine, but did not affect the sensitivity to cisplatin or etoposide in SNU638. In the Western blot analysis, RhoA decreased the PARP cleavage, which was accompanied by a concurrent reduction in cell death. The gene expression profile after a cDNA microarray analysis demonstrated that RhoA was associated with the differential expression of 19 genes, including those involved in anti-oxidant defense, glucose metabolism, anti-apoptosis and protein turnover. CONCLUSION: Gastric cancer cells with a high expression of RhoA could be resistant to chemotherapeutic drugs, such as taxol or vincristine, implying that treatment strategies aimed at inactivation of RhoA might be promising for improving the efficacy of these chemotherapeutic drugs.
Keyword
MeSH Terms
-
Actin Cytoskeleton
Blotting, Western
Cell Death
Cell Line
Cisplatin
Etoposide
Fluorouracil
Glucose
Lovastatin
Metabolism
Microarray Analysis
Oligonucleotide Array Sequence Analysis
Paclitaxel
Stomach Neoplasms*
Transcriptome
Transducers
Vincristine
Cisplatin
Etoposide
Fluorouracil
Glucose
Lovastatin
Paclitaxel
Vincristine
Figure
Reference
-
1. Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991; 349:117–127. PMID: 1898771.
Article2. Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995; 15:6443–6453. PMID: 7565796.
Article3. Moorman JP, Bobak DA, Hahn CS. Inactivation of the small GTP binding protein Rho induces multinucleate cell formation and apoptosis in murine T lymphoma EL4. J Immunol. 1996; 156:4146–4153. PMID: 8666781.4. Perona R, Esteve P, Jimenez B, Ballestero RP, Ramony Cajal S, Lacal JC. Tumorigenic activity of rho genes from Aplysia californica. Oncogene. 1993; 8:1285–1292. PMID: 8479750.5. Prendergast GC, Khosravi-Far R, Solski PA, Kurzawa H, Lebowitz PF, Der CJ. Critical role of Rho in cell transformation by oncogenic Ras. Oncogene. 1995; 10:2289–2296. PMID: 7784077.6. Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res. 1999; 59:2004–2010. PMID: 10213513.7. Moscow JA, He R, Gnarra JR, Knusten T, Weng Y, Zhao WP, et al. Examination of human tumors for rhoA mutations. Oncogene. 1994; 9:189–194. PMID: 8302578.8. Bobak D, Moorman J, Guanzon A, Gilmer L, Hahn C. Inactivation of the small GTPase Rho disrupts cellular attachment and induces adhesion-dependent and adhesion-independent apoptosis. Oncogene. 1997; 15:2179–2189. PMID: 9393976.
Article9. Esteve P, del Peso L, Lacal JC. Induction of apoptosis by rho in NIH 3T3 cells requires two complementary signals. Ceramides function as a progression factor for apoptosis. Oncogene. 1995; 11:2657–2665. PMID: 8545123.10. Carson JP, Kulik G, Weber MJ. Antiapoptotic signaling in LNCaP prostate cancer cells: a survival signaling pathway independent of phosphatidylinositol 3'-kinase and Akt/protein kinase B. Cancer Res. 1999; 59:1449–1453. PMID: 10197612.11. Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3'-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human gastric carcinoma cells. Cancer Res. 1999; 59:2891–2897. PMID: 10383151.12. Kang HC, Kim IJ, Park JH, Shin Y, Ku JL, Jung MS, et al. Identification of genes with differential expression in acquired drug-resistant gastric cancer cells using high-density oligonucleotide microarrays. Clin Cancer Res. 2004; 10:272–284. PMID: 14734480.
Article13. Moorehead RA, Singh G. Influence of the proto-oncogene c-fos on cisplatin sensitivity. Biochem Pharmacol. 2000; 59:337–345. PMID: 10644041.
Article14. Hettinga JV, Lemstra W, Meijer C, Los G, de Vries EG, Konings AW, et al. Heat-shock protein expression in cisplatinsensitive and -resistant human tumor cells. Int J Cancer. 1996; 67:800–807. PMID: 8824551.
Article15. Mizutani Y, Fukumoto M, Bonavida B, Yoshida O. Enhancement of sensitivity of urinary bladder tumor cells to cisplatin by c-myc antisense oligonucleotide. Cancer. 1994; 74:2546–2554. PMID: 7923012.
Article16. Beale PJ, Rogers P, Boxall F, Sharp SY, Kelland LR. BCL-2 family protein expression and platinum drug resistance in ovarian carcinoma. Br J Cancer. 2000; 82:436–440. PMID: 10646901.
Article17. Park C, Lee I, Kang WK. Lovastatin-induced E2F-1 modulation and its effect on prostate cancer cell death. Carcinogenesis. 2001; 22:1727–1731. PMID: 11577016.
Article18. Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ. Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer. 2001; 85:273–278. PMID: 11461089.
Article19. Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000; 275:14624–14631. PMID: 10799549.
Article20. Scheffer GL, Wijngaard PL, Flens MJ, Izquierdo MA, Slovak ML, Pinedo HM, et al. The drug resistance-related protein LRP is the human major vault protein. Nat Med. 1995; 1:578–582. PMID: 7585126.
Article21. Toffoli G, Frustaci S, Tumiotto L, Talamini R, Gherlinzoni F, Picci P, et al. Expression of MDR1 and GST-pi in human soft tissue sarcomas: relation to drug resistance and biological aggressiveness. Ann Oncol. 1992; 3:63–69. PMID: 1606072.22. Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000; 407:810–816. PMID: 11048733.
Article23. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990; 343:425–430. PMID: 1967820.
Article24. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998; 281:1680–1683. PMID: 9733516.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ceramide Synthase 6 Mediates TripleNegative Breast Cancer Response to Chemotherapy Through RhoA- and EGFR-Mediated Signaling Pathways
- Chemoresistance in ovarian cancer: exploiting cancer stem cell metabolism
- Characterization of the Rho GTPase-Activating Protein RhoGAP68F
- Hyaluronan-mediated motility receptor-mediated aerobic glycolysis enhances stem-like properties and chemoresistance in lung adenocarcinoma
- Sensitization of 5-Fluorouracil-Resistant SNUC5 Colon Cancer Cells to Apoptosis by α-Mangostin