Endocrinol Metab.
2014 Sep;29(3):280-292. 10.3803/EnM.2014.29.3.280.
Early Prediction of Long-Term Response to Cabergoline in Patients with Macroprolactinomas
- Affiliations
-
- 1Division of Endocrinology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea. hjwcarrot@paik.ac.kr
- KMID: 2169464
- DOI: http://doi.org/10.3803/EnM.2014.29.3.280
Abstract
- BACKGROUND
Cabergoline is typically effective for treating prolactinomas; however, some patients display cabergoline resistance, and the early characteristics of these patients remain unclear. We analyzed early indicators predicting long-term response to cabergoline.
METHODS
We retrospectively reviewed the cases of 44 patients with macroprolactinomas who received cabergoline as first-line treatment; the patients were followed for a median of 16 months. The influence of various clinical parameters on outcomes was evaluated.
RESULTS
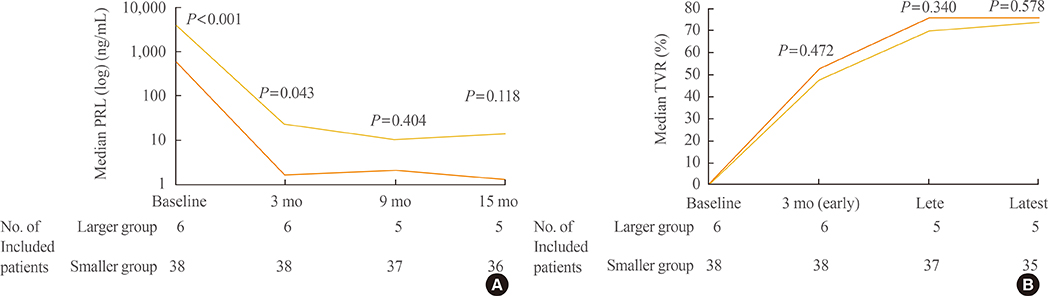
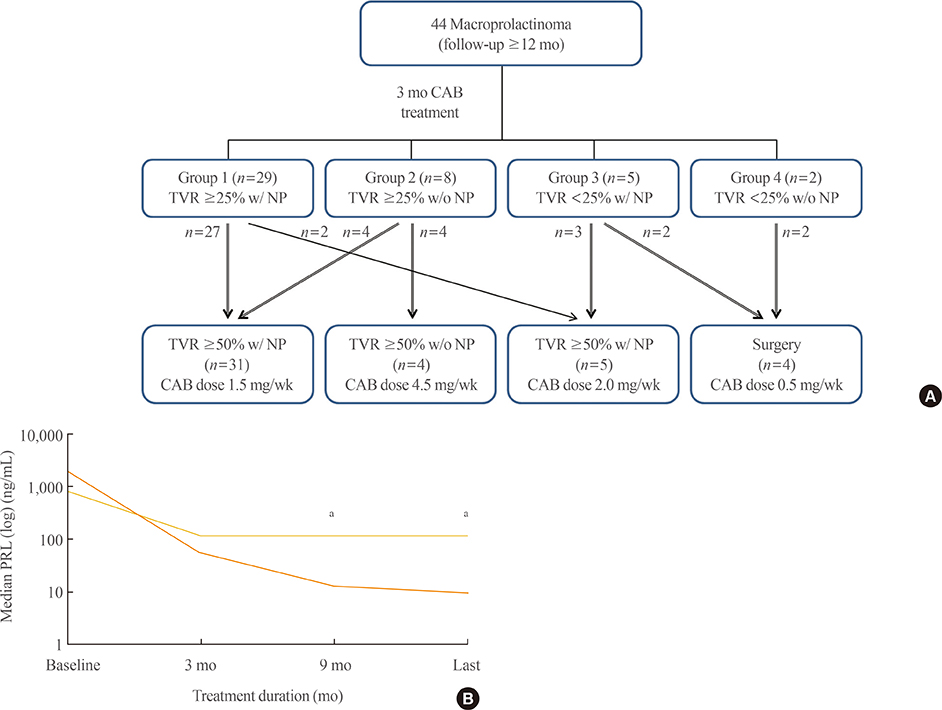
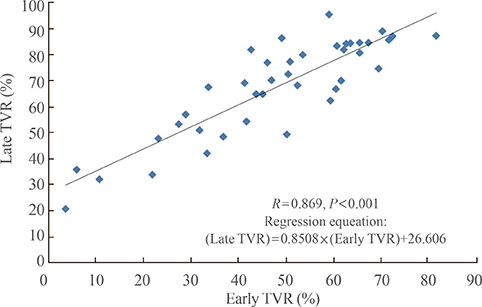
Forty patients (90.9%) were treated medically and displayed tumor volume reduction (TVR) of 74.7%, a prolactin normalization (NP) rate of 81.8%, and a complete response (CR; TVR >50% with NP, without surgery) rate of 70.5%. Most patients (93.1%) with TVR > or =25% and NP at 3 months eventually achieved CR, whereas only 50% of patients with TVR > or =25% without NP and no patients with TVR <25% achieved CR. TVR at 3 months was strongly correlated with final TVR (R=0.785). Patients with large macroadenomas exhibited a low NP rate at 3 months, but eventually achieved TVR and NP rates similar to those of patients with smaller tumors. Surgery independently reduced the final dose of cabergoline (beta=-1.181 mg/week), and two of four patients who underwent surgery were able to discontinue cabergoline.
CONCLUSION
Determining cabergoline response using TVR and NP 3 months after treatment is useful for predicting later outcomes. However, further cabergoline administration should be considered for patients with TVR >25% at 3 months without NP, particularly those with huge prolactinomas, because a delayed response may be achieved. As surgery can reduce the cabergoline dose necessary for successful disease control, it should be considered for cabergoline-resistant patients.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Elucidating Clinical Queries for Tailored Therapy in Patients with Prolactinoma
Min-Ho Lee, Jae Won Hong, Kyungwon Kim, Cheol Ryong Ku, Eun Jig Lee
Endocrinol Metab. 2024;39(6):819-826. doi: 10.3803/EnM.2024.2057.
Reference
-
1. Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006; 27:485–534.2. Colao A. Pituitary tumours: the prolactinoma. Best Pract Res Clin Endocrinol Metab. 2009; 23:575–596.3. Colao A, Lombardi G. Growth-hormone and prolactin excess. Lancet. 1998; 352:1455–1461.4. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006; 91:4769–4775.5. Oh MC, Kunwar S, Blevins L, Aghi MK. Medical versus surgical management of prolactinomas. Neurosurg Clin N Am. 2012; 23:669–678.6. Colao A, Savastano S. Medical treatment of prolactinomas. Nat Rev Endocrinol. 2011; 7:267–278.7. Di Sarno A, Landi ML, Cappabianca P, Di Salle F, Rossi FW, Pivonello R, Di Somma C, Faggiano A, Lombardi G, Colao A. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001; 86:5256–5261.8. Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A, Fahlbusch R, Fideleff H, Hadani M, Kelly P, Kleinberg D, Laws E, Marek J, Scanlon M, Sobrinho LG, Wass JA, Giustina A. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006; 65:265–273.9. Delgrange E, Maiter D, Donckier J. Effects of the dopamine agonist cabergoline in patients with prolactinoma intolerant or resistant to bromocriptine. Eur J Endocrinol. 1996; 134:454–456.10. Colao A, Di Sarno A, Sarnacchiaro F, Ferone D, Di Renzo G, Merola B, Annunziato L, Lombardi G. Prolactinomas resistant to standard dopamine agonists respond to chronic cabergoline treatment. J Clin Endocrinol Metab. 1997; 82:876–883.11. Molitch ME. Pharmacologic resistance in prolactinoma patients. Pituitary. 2005; 8:43–52.12. Primeau V, Raftopoulos C, Maiter D. Outcomes of transsphenoidal surgery in prolactinomas: improvement of hormonal control in dopamine agonist-resistant patients. Eur J Endocrinol. 2012; 166:779–786.13. Ono M, Miki N, Kawamata T, Makino R, Amano K, Seki T, Kubo O, Hori T, Takano K. Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J Clin Endocrinol Metab. 2008; 93:4721–4727.14. Ono M, Miki N, Amano K, Kawamata T, Seki T, Makino R, Takano K, Izumi S, Okada Y, Hori T. Individualized high-dose cabergoline therapy for hyperprolactinemic infertility in women with micro- and macroprolactinomas. J Clin Endocrinol Metab. 2010; 95:2672–2679.15. Vroonen L, Jaffrain-Rea ML, Petrossians P, Tamagno G, Chanson P, Vilar L, Borson-Chazot F, Naves LA, Brue T, Gatta B, Delemer B, Ciccarelli E, Beck-Peccoz P, Caron P, Daly AF, Beckers A. Prolactinomas resistant to standard doses of cabergoline: a multicenter study of 92 patients. Eur J Endocrinol. 2012; 167:651–662.16. Yang MS, Hong JW, Lee SK, Lee EJ, Kim SH. Clinical management and outcome of 36 invasive prolactinomas treated with dopamine agonist. J Neurooncol. 2011; 104:195–204.17. Di Chiro G, Nelson KB. The volume of the sella turcica. Am J Roentgenol Radium Ther Nucl Med. 1962; 87:989–1008.18. Frieze TW, Mong DP, Koops MK. "Hook effect" in prolactinomas: case report and review of literature. Endocr Pract. 2002; 8:296–303.19. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA. Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:273–288.20. Cho EH, Lee SA, Chung JY, Koh EH, Cho YH, Kim JH, Kim CJ, Kim MS. Efficacy and safety of cabergoline as first line treatment for invasive giant prolactinoma. J Korean Med Sci. 2009; 24:874–878.21. Iglesias P, Diez JJ. Macroprolactinoma: a diagnostic and therapeutic update. QJM. 2013; 106:495–504.22. Verhelst J, Abs R, Maiter D, van den Bruel A, Vandeweghe M, Velkeniers B, Mockel J, Lamberigts G, Petrossians P, Coremans P, Mahler C, Stevenaert A, Verlooy J, Raftopoulos C, Beckers A. Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab. 1999; 84:2518–2522.23. Colao A, Di Sarno A, Landi ML, Scavuzzo F, Cappabianca P, Pivonello R, Volpe R, Di Salle F, Cirillo S, Annunziato L, Lombardi G. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: a prospective study in 110 patients. J Clin Endocrinol Metab. 2000; 85:2247–2252.24. Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007; 356:29–38.25. Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med. 2007; 356:39–46.26. Corvol JC, Anzouan-Kacou JB, Fauveau E, Bonnet AM, Lebrun-Vignes B, Girault C, Agid Y, Lechat P, Isnard R, Lacomblez L. Heart valve regurgitation, pergolide use, and parkinson disease: an observational study and meta-analysis. Arch Neurol. 2007; 64:1721–1726.27. Antonini A, Poewe W. Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson's disease. Lancet Neurol. 2007; 6:826–829.28. Valassi E, Klibanski A, Biller BM. Clinical review: potential cardiac valve effects of dopamine agonists in hyperprolactinemia. J Clin Endocrinol Metab. 2010; 95:1025–1033.29. Colao A, Galderisi M, Di Sarno A, Pardo M, Gaccione M, D'Andrea M, Guerra E, Pivonello R, Lerro G, Lombardi G. Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocrinol Metab. 2008; 93:3777–3784.30. Wakil A, Rigby AS, Clark AL, Kallvikbacka-Bennett A, Atkin SL. Low dose cabergoline for hyperprolactinaemia is not associated with clinically significant valvular heart disease. Eur J Endocrinol. 2008; 159:R11–R14.31. Saeki N, Nakamura M, Sunami K, Yamaura A. Surgical indication after bromocriptine therapy on giant prolactinomas: effects and limitations of the medical treatment. Endocr J. 1998; 45:529–537.32. Menucci M, Quinones-Hinojosa A, Burger P, Salvatori R. Effect of dopaminergic drug treatment on surgical findings in prolactinomas. Pituitary. 2011; 14:68–74.33. Regal M, Paramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf). 2001; 55:735–740.34. Dekkers OM, Hammer S, de Keizer RJ, Roelfsema F, Schutte PJ, Smit JW, Romijn JA, Pereira AM. The natural course of non-functioning pituitary macroadenomas. Eur J Endocrinol. 2007; 156:217–224.35. Karavitaki N, Collison K, Halliday J, Byrne JV, Price P, Cudlip S, Wass JA. What is the natural history of nonoperated nonfunctioning pituitary adenomas? Clin Endocrinol (Oxf). 2007; 67:938–943.36. Iglesias P, Bernal C, Villabona C, Castro JC, Arrieta F, Diez JJ. Prolactinomas in men: a multicentre and retrospective analysis of treatment outcome. Clin Endocrinol (Oxf). 2012; 77:281–287.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update in Prolactinomas
- Efficacy and Safety of Cabergoline as First Line Treatment for Invasive Giant Prolactinoma
- Complementary health education and clinical guidance for treating women experiencing infertility along with unexplained resistant hyperprolactinemia
- Does the timing of cabergoline administration impact rates of ovarian hyperstimulation syndrome?
- Long-term outcomes of a 5-year follow up of patients with immune thrombocytopenic purpura after splenectomy