J Korean Med Sci.
2009 Oct;24(5):874-878. 10.3346/jkms.2009.24.5.874.
Efficacy and Safety of Cabergoline as First Line Treatment for Invasive Giant Prolactinoma
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. mskim@amc.seoul.kr
- 2Department of Neurosurgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1782009
- DOI: http://doi.org/10.3346/jkms.2009.24.5.874
Abstract
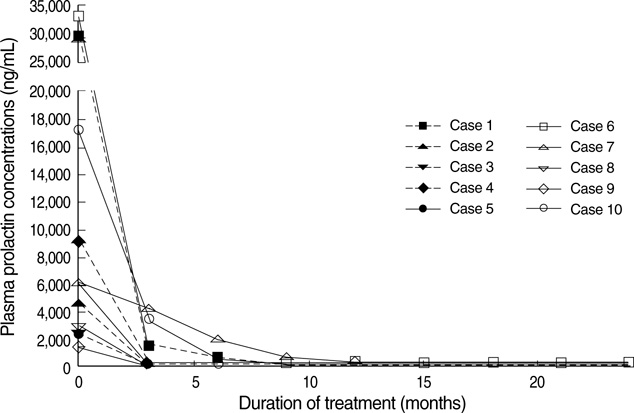
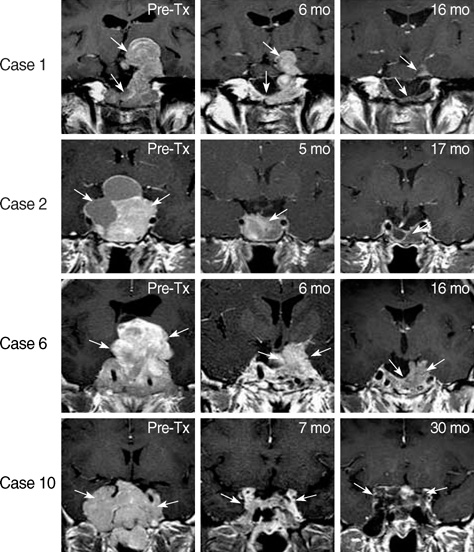
- Although cabergoline is effective in the treatment of micro- and macro-prolactinoma, little is known about its efficacy in the treatment of invasive giant prolactinoma. We investigated the efficacy and safety of cabergoline in 10 male patients with invasive giant prolactinoma. Before treatment, mean serum prolactin level was 11,426 ng/mL (range, 1,450-33,200 ng/mL) and mean maximum tumor diameter was 51 mm (range, 40-77 mm). Three months after initiation of cabergoline treatment, serum prolactin concentrations decreased more than 97% in 9 patients; at last follow-up (mean treatment duration, 19 months), the mean decrease in serum prolactin concentrations was 98%, with 5 patients having normal serum prolactin levels. At first MRI follow-up (3-12 months after initiation of cabergoline), the mean reduction in tumor size was 85+/-4% (range, 57-98%). Cabergoline treatment for more than 12 months caused a greater reduction in tumor size compared to the treatment for less than 12 months (97+/-1% vs. 78+/-7%, P<0.05). These findings indicate that cabergoline treatment led to a significant and rapid reduction in serum prolactin concentrations and tumor size in patients with giant prolactinoma. Therefore, cabergoline represents an effective and well-tolerated treatment for invasive giant prolactinoma.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Bromocriptine Therapy for the Treatment of Invasive Prolactinoma: The Single Institute Experience
Kyung Rae Cho, Kyung-Il Jo, Hyung Jin Shin
Brain Tumor Res Treat. 2013;1(2):71-77. doi: 10.14791/btrt.2013.1.2.71.
Reference
-
1. Shrivastava RK, Arginteanu MS, King WA, Post KD. Giant prolactinomas: clinical management and long-term follow up. J Neurosurg. 2002. 97:299–306.
Article2. Shimon I, Benbassat C, Hadani M. Effectiveness of long-term cabergoline treatment for giant prolactinoma: study of 12 men. Eur J Endocrinol. 2007. 156:225–231.
Article3. Schaller B. Gender-related differences in prolactinomas. A clinicopathological study. Neuro Endocrinol Lett. 2005. 26:152–159.4. Biller BM, Molitch ME, Vance ML, Cannistraro KB, Davis KR, Simons JA, Schoenfelder JR, Klibanski A. Treatment of prolactin-secreting macroadenomas with the once-weekly dopamine agonist cabergoline. J Clin Endocrinol Metab. 1996. 81:2338–2343.
Article5. Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J. Sex-related difference in the growth of prolactinomas: a clinical and proliferation marker study. J Clin Endocrinol Metab. 1997. 82:2102–2107.
Article6. Hulting AL, Muhr C, Lundberg PO, Werner S. Prolactinomas in men: clinical characteristics and the effect of bromocriptine treatment. Acta Med Scand. 1985. 217:101–109.
Article7. Ferrari CI, Abs R, Bevan JS, Brabant G, Ciccarelli E, Motta T, Mucci M, Muratori M, Musatti L, Verbessem G, Scanlon MF. Treatment of macroprolactinoma with cabergoline: a study of 85 patients. Clin Endocrinol (Oxf). 1997. 46:409–413.
Article8. Colao A, Vitale G, Cappabianca P, Briganti F, Ciccarelli A, De Rosa M, Zarrilli S, Lombardi G. Outcome of cabergoline treatment in men with prolactinoma: effects of a 24-month treatment on prolactin levels, tumor mass, recovery of pituitary function, and semen analysis. J Clin Endocrinol Metab. 2004. 89:1704–1711.
Article9. Corsello SM, Ubertini G, Altomare M, Lovicu RM, Migneco MG, Rota CA, Colosimo C. Giant prolactinomas in men: efficacy of cabergoline treatment. Clin Endocrinol (Oxf). 2003. 58:662–670.
Article10. Ciccarelli E, Camanni F. Diagnosis and drug therapy of prolactinoma. Drugs. 1996. 51:954–965.
Article11. Suliman SG, Gurlek A, Byrne JV, Sullivan N, Thanabalasingham G, Cudlip S, Ansorge O, Wass JA. Nonsurgical cerebrospinal fluid rhinorrhea in invasive macroprolactinoma: incidence, radiological, and clinicopathological features. J Clin Endocrinol Metab. 2007. 92:3829–3835.
Article12. Leong KS, Foy PM, Swift AC, Atkin SL, Hadden DR, MacFarlane IA. CSF rhinorrhoea following treatment with dopamine agonists for massive invasive prolactinomas. Clin Endocrinol (Oxf). 2000. 52:43–49.
Article13. Baskin DS, Wilson CB. CSF rhinorrhea after bromocriptine for prolactinoma. N Engl J Med. 1982. 306:178.
Article14. Serri O, Rasio E, Beauregard H, Hardy J, Somma M. Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N Engl J Med. 1983. 309:280–283.
Article15. Nelson PB, Goodman M, Maroon JC, Martinez AJ, Moossy J, Robinson AG. Factors in predicting outcome from operation in patients with prolactin-secreting pituitary adenomas. Neurosurgery. 1983. 13:634–641.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update in Prolactinomas
- Early Prediction of Long-Term Response to Cabergoline in Patients with Macroprolactinomas
- Bromocriptine Therapy for the Treatment of Invasive Prolactinoma: The Single Institute Experience
- Multiple Endocrine Neoplasia Type 1 Presenting with an Invasive Giant Prolactinoma
- Prolactinoma presenting as giant cystic pituitary mass