Endocrinol Metab.
2013 Dec;28(4):288-296. 10.3803/EnM.2013.28.4.288.
Effects of Chronic Restraint Stress on Body Weight, Food Intake, and Hypothalamic Gene Expressions in Mice
- Affiliations
-
- 1Department of Anatomy and Neurobiology, Institute of Health Sciences, Medical Research Center for Neural Dysfunction, Gyeongsang National University School of Medicine, Jinju, Korea. kangss@gnu.ac.kr
- KMID: 2169307
- DOI: http://doi.org/10.3803/EnM.2013.28.4.288
Abstract
- BACKGROUND
Stress affects body weight and food intake, but the underlying mechanisms are not well understood.
METHODS
We evaluated the changes in body weight and food intake of ICR male mice subjected to daily 2 hours restraint stress for 15 days. Hypothalamic gene expression profiling was analyzed by cDNA microarray.
RESULTS
Daily body weight and food intake measurements revealed that both parameters decreased rapidly after initiating daily restraint stress. Body weights of stressed mice then remained significantly lower than the control body weights, even though food intake slowly recovered to 90% of the control intake at the end of the experiment. cDNA microarray analysis revealed that chronic restraint stress affects the expression of hypothalamic genes possibly related to body weight control. Since decreases of daily food intake and body weight were remarkable in days 1 to 4 of restraint, we examined the expression of food intake-related genes in the hypothalamus. During these periods, the expressions of ghrelin and pro-opiomelanocortin mRNA were significantly changed in mice undergoing restraint stress. Moreover, daily serum corticosterone levels gradually increased, while leptin levels significantly decreased.
CONCLUSION
The present study demonstrates that restraint stress affects body weight and food intake by initially modifying canonical food intake-related genes and then later modifying other genes involved in energy metabolism. These genetic changes appear to be mediated, at least in part, by corticosterone.
Keyword
MeSH Terms
-
Animals
Body Weight*
Corticosterone
DNA, Complementary
Eating*
Energy Metabolism
Gene Expression Profiling
Gene Expression*
Ghrelin
Humans
Hypothalamus
Leptin
Male
Mice*
Oligonucleotide Array Sequence Analysis
Pro-Opiomelanocortin
RNA, Messenger
Corticosterone
DNA, Complementary
Ghrelin
Leptin
Pro-Opiomelanocortin
RNA, Messenger
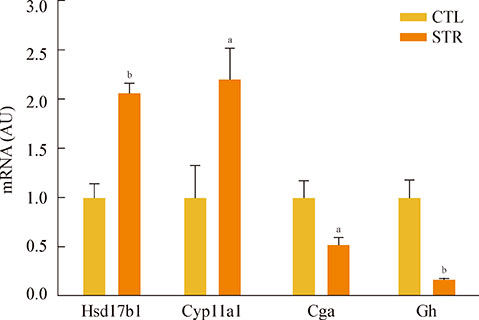
Figure
Cited by 1 articles
-
Brief Review of Articles in '
Endocrinology and Metabolism ' in 2013
Won-Young Lee
Endocrinol Metab. 2014;29(3):251-256. doi: 10.3803/EnM.2014.29.3.251.
Reference
-
1. Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994; 18:223–249.2. Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990; 27:1094–1102.3. Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1992; 4:517–526.4. Woods SC, Seeley RJ, Porte D Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998; 280:1378–1383.5. Korner J, Savontaus E, Chua SC Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol. 2001; 13:959–966.6. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000; 407:908–913.7. van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004; 25:426–457.8. Proulx K, Richard D, Walker CD. Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology. 2002; 143:4683–4692.9. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996; 382:250–252.10. Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998; 1:445–450.11. Smagin GN, Howell LA, Redmann S Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999; 276(5 Pt 2):R1461–R1468.12. Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994; 55:747–753.13. Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998; 275(6 Pt 2):R1928–R1938.14. Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C. Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int. 2003; 42:107–114.15. Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006; 18:13–24.16. Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. C hronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav. 2006; 49:615–625.17. Ottenweller JE, Servatius RJ, Tapp WN, Drastal SD, Bergen MT, Natelson BH. A chronic stress state in rats: effects of repeated stress on basal corticosterone and behavior. Physiol Behav. 1992; 51:689–698.18. Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002; 115:229–242.19. Strekalova T, Spanagel R, Dolgov O, Bartsch D. Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol. 2005; 16:171–180.20. Ricart-Jane D, Cejudo-Martin P, Peinado-Onsurbe J, Lopez-Tejero MD, Llobera M. Changes in lipoprotein lipase modulate tissue energy supply during stress. J Appl Physiol (1985). 2005; 99:1343–1351.21. Sato T, Yamamoto H, Sawada N, Nashiki K, Tsuji M, Muto K, Kume H, Sasaki H, Arai H, Nikawa T, Taketani Y, Takeda E. Restraint stress alters the duodenal expression of genes important for lipid metabolism in rat. Toxicology. 2006; 227:248–261.22. Allen DL, McCall GE, Loh AS, Madden MC, Mehan RS. Acute daily psychological stress causes increased atrophic gene expression and myostatin-dependent muscle atrophy. Am J Physiol Regul Integr Comp Physiol. 2010; 299:R889–R898.23. Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002; 143:155–162.24. Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003; 52:948–956.25. Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003; 37:649–661.26. Chan CB, Cheng CH. Identification and functional characterization of two alternatively spliced growth hormone secretagogue receptor transcripts from the pituitary of black seabream Acanthopagrus schlegeli. Mol Cell Endocrinol. 2004; 214:81–95.27. Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav. 2007; 92:263–271.28. Harris RB, Mitchell TD, Simpson J, Redmann SM Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol. 2002; 282:R77–R88.29. Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005; 30:939–946.30. Marti O, Harbuz MS, Andres R, Lightman SL, Armario A. Activation of the hypothalamic-pituitary axis in adrenalectomised rats: potentiation by chronic stress. Brain Res. 1999; 821:1–7.31. Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998; 84:1025–1039.32. Dal-Zotto S, Marti O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav Brain Res. 2000; 114:175–181.33. Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001; 26:443–459.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic Restraint Stress Decreases the Excitability of Hypothalamic POMC Neuron and Increases Food Intake
- The Effects of Metformin Given into the Brain on Food Intake and a Expressions of Hypothalamic Neurotransmitters in the Rats
- High-fat Intake is Associated with Alteration of Peripheral Circadian Clock Gene Expression
- Herpes Virus Entry Mediator Signaling in the Brain Is Imperative in Acute Inflammation-Induced Anorexia and Body Weight Loss
- Increase of NADPH-diaphorase Expression in Hypothalamus of Stat4 Knockout Mice