Endocrinol Metab.
2013 Sep;28(3):214-220. 10.3803/EnM.2013.28.3.214.
Herpes Virus Entry Mediator Signaling in the Brain Is Imperative in Acute Inflammation-Induced Anorexia and Body Weight Loss
- Affiliations
-
- 1Department of Biological Sciences, University of Ulsan College of Natural Sciences, Ulsan, Korea. bjlee@ulsan.ac.kr
- KMID: 1973826
- DOI: http://doi.org/10.3803/EnM.2013.28.3.214
Abstract
- BACKGROUND
Reduced appetite and body weight loss are typical symptoms of inflammatory diseases. A number of inflammatory stimuli are responsible for the imbalance in energy homeostasis, leading to metabolic disorders. The herpes virus entry mediator (HVEM) protein plays an important role in the development of various inflammatory diseases, such as intestinal inflammation and diet-induced obesity. However, the role of HVEM in the brain is largely unknown. This study aims to investigate whether HVEM signaling in the brain is involved in inflammation-induced anorexia and body weight loss.
METHODS
Food intake and body weight were measured at 24 hours after intraperitoneal injection of lipopolysaccharide (LPS) or intracerebroventricular injection of recombinant mouse LIGHT (also called tumor necrosis factor receptor superfamily 14, TNFSF14), an HVEM ligand, into 8- to 10-week-old male C57BL/6 mice and mice lacking HVEM expression (HVEM-/-). We also assessed LPS-induced change in hypothalamic expression of HVEM using immunohistochemistry.
RESULTS
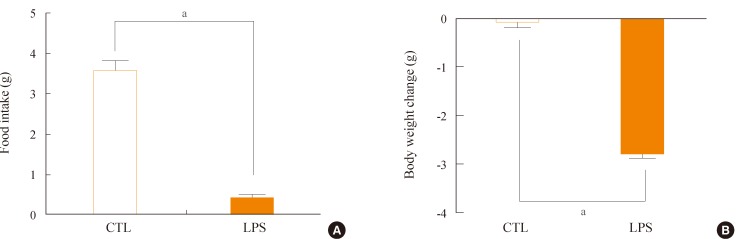
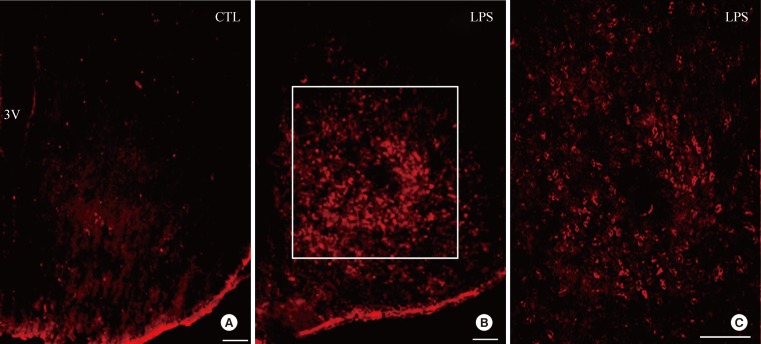
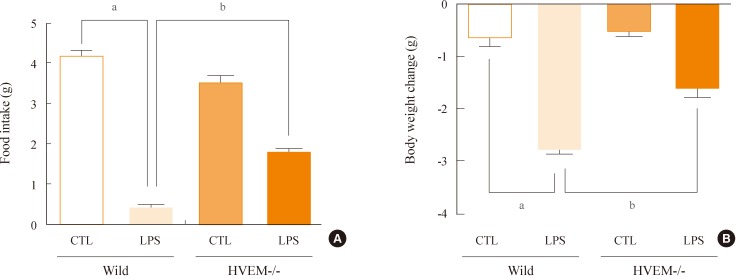
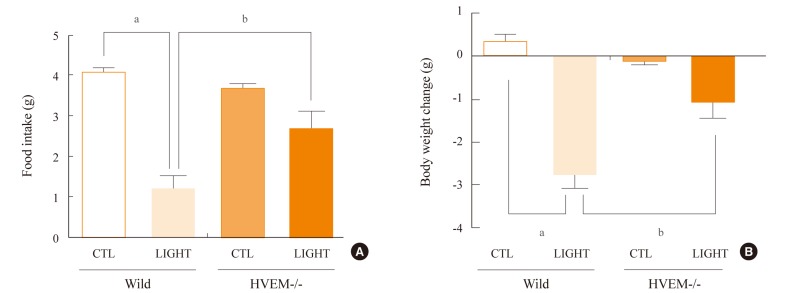
Administration of LPS significantly reduced food intake and body weight, and moreover, increased expression of HVEM in the hypothalamic arcuate nucleus. However, LPS induced only minor decreases in food intake and body weight in HVEM-/- mice. Administration of LIGHT into the brain was very effective at decreasing food intake and body weight in wild-type mice, but was less effective in HVEM-/- mice.
CONCLUSION
Activation of brain HVEM signaling is responsible for inflammation-induced anorexia and body weight loss.
MeSH Terms
Figure
Cited by 1 articles
-
Brief Review of Articles in '
Endocrinology and Metabolism ' in 2013
Won-Young Lee
Endocrinol Metab. 2014;29(3):251-256. doi: 10.3803/EnM.2014.29.3.251.
Reference
-
1. Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988; 12:123–137. PMID: 3050629.
Article2. Plata-Salaman CR. Anorexia during acute and chronic disease. Nutrition. 1996; 12:69–78. PMID: 8724375.
Article3. Szelenyi Z, Szekely M. Sickness behavior in fever and hypothermia. Front Biosci. 2004; 9:2447–2456. PMID: 15353297.4. Matthys P, Billiau A. Cytokines and cachexia. Nutrition. 1997; 13:763–770. PMID: 9290087.
Article6. Grinspoon S, Mulligan K. Department of Health and Human Services Working Group on the Prevention and Treatment of Wasting and Weight Loss. Weight loss and wasting in patients infected with human immunodeficiency virus. Clin Infect Dis. 2003; 36(Suppl 2):S69–S78. PMID: 12652374.
Article7. Argiles JM, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev. 1999; 19:223–248. PMID: 10232651.8. Langhans W. Anorexia of infection: current prospects. Nutrition. 2000; 16:996–1005. PMID: 11054606.
Article9. McNamara MJ, Alexander HR, Norton JA. Cytokines and their role in the pathophysiology of cancer cachexia. JPEN J Parenter Enteral Nutr. 1992; 16(6 Suppl):50S–55S. PMID: 1287224.
Article10. Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun. 2008; 22:838–849. PMID: 18255258.11. Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005; 19:1329–1331. PMID: 15919760.12. Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991; 71:93–127. PMID: 1986393.
Article13. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008; 9:46–56. PMID: 18073775.
Article14. Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012; 37:1491–1505. PMID: 22386198.
Article15. Hines DJ, Choi HB, Hines RM, Phillips AG, MacVicar BA. Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS One. 2013; 8:e60388. PMID: 23555964.
Article16. Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995; 85:3378–3404. PMID: 7780126.
Article17. Shui JW, Steinberg MW, Kronenberg M. Regulation of inflammation, autoimmunity, and infection immunity by HVEM-BTLA signaling. J Leukoc Biol. 2011; 89:517–523. PMID: 21106644.
Article18. Steinberg MW, Shui JW, Ware CF, Kronenberg M. Regulating the mucosal immune system: the contrasting roles of LIGHT, HVEM, and their various partners. Semin Immunopathol. 2009; 31:207–221. PMID: 19495760.
Article19. Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009; 229:244–258. PMID: 19426226.
Article20. Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997; 272:14029–14032. PMID: 9162022.21. Schaer C, Hiltbrunner S, Ernst B, Mueller C, Kurrer M, Kopf M, Harris NL. HVEM signalling promotes colitis. PLoS One. 2011; 6:e18495. PMID: 21533159.
Article22. Wang J, Anders RA, Wang Y, Turner JR, Abraham C, Pfeffer K, Fu YX. The critical role of LIGHT in promoting intestinal inflammation and Crohn’s disease. J Immunol. 2005; 174:8173–8182. PMID: 15944326.
Article23. An MM, Fan KX, Cao YB, Shen H, Zhang JD, Lu L, Gao PH, Jiang YY. Lymphtoxin beta receptor-Ig protects from T-cell-mediated liver injury in mice through blocking LIGHT/HVEM signaling. Biol Pharm Bull. 2006; 29:2025–2030. PMID: 17015945.
Article24. Kim HJ, Kim HM, Kim CS, Jeong CS, Choi HS, Kawada T, Kim BS, Yu R. HVEM-deficient mice fed a high-fat diet are protected from adipose tissue inflammation and glucose intolerance. FEBS Lett. 2011; 585:2285–2290. PMID: 21679708.
Article25. Kim WK, Park JS, Sul OJ, Seo JH, Choi BK, Park HY, Latour AM, Koller BH, Kwon BS, Jeong CS. Role of TNFR-related 2 mediated immune responses in dextran sulfate sodium-induced inflammatory bowel disease. Mol Cells. 2011; 31:99–104. PMID: 21347711.
Article26. Kim HM, Jeong CS, Choi HS, Kawada T, Yu R. LIGHT/TNFSF14 enhances adipose tissue inflammatory responses through its interaction with HVEM. FEBS Lett. 2011; 585:579–584. PMID: 21236258.
Article27. Langhans W. Signals generating anorexia during acute illness. Proc Nutr Soc. 2007; 66:321–330. PMID: 17637084.
Article28. Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995; 270:16483–16486. PMID: 7622446.
Article29. Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013; 61:71–90. PMID: 22674585.
Article30. Elmquist JK, Flier JS. Neuroscience. The fat-brain axis enters a new dimension. Science. 2004; 304:63–64. PMID: 15064411.31. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006; 443:289–295. PMID: 16988703.
Article32. Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002; 25:154–159. PMID: 11852148.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anorexia Nervosa with Autonomic Dysfunction
- Sacral Herpes Zoster Associated with Voiding Dysfunction in a Young Patient with Scrub Typhus
- A Case of Human Immunodeficiency Virus/Hepatitis B Virus Coinfection with Persistent Anorexia and Weight Loss as Early Clinical Symptom
- Effects of pretreatment of serotonin synthesis inhibitor p-chlorophenylalanine on lipopolysaccharide-induced anorexia in rats
- Impact of Body Mass Index on Acute Pain and Postherpetic Neuralgia of Patients with Herpes Zoster