Immune Netw.
2014 Apr;14(2):107-115. 10.4110/in.2014.14.2.107.
Phellinus linteus Extract Exerts Anti-asthmatic Effects by Suppressing NF-kappaB and p38 MAPK Activity in an OVA-induced Mouse Model of Asthma
- Affiliations
-
- 1Department of Anatomy and Histology and Embryology, Yanbian University School of Basic Medical Sciences, YanJi 133002, Jilin, China.
- 2Department of Anatomy, Medical School, Institute for Medical Sciences, Chonbuk National University, Jeonju 561-180, Korea. why76@jbnu.ac.kr
- KMID: 2168021
- DOI: http://doi.org/10.4110/in.2014.14.2.107
Abstract
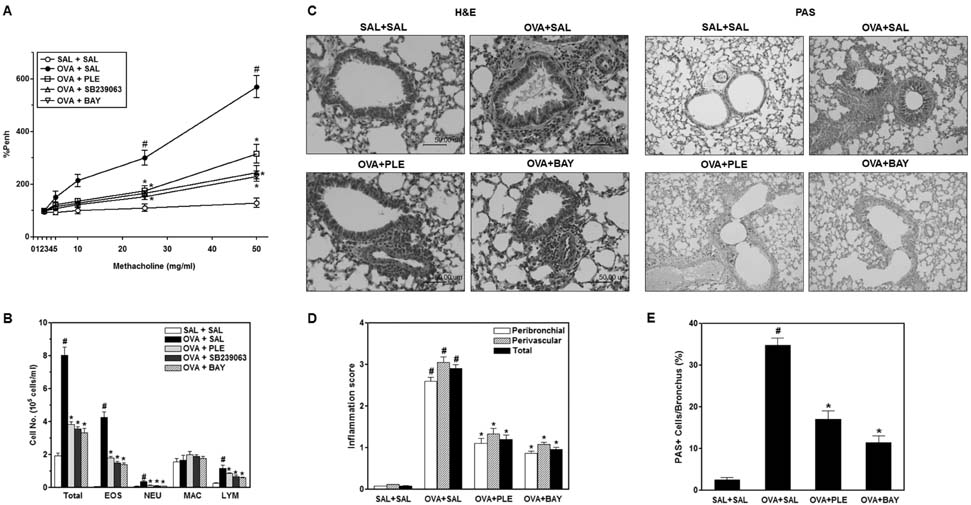
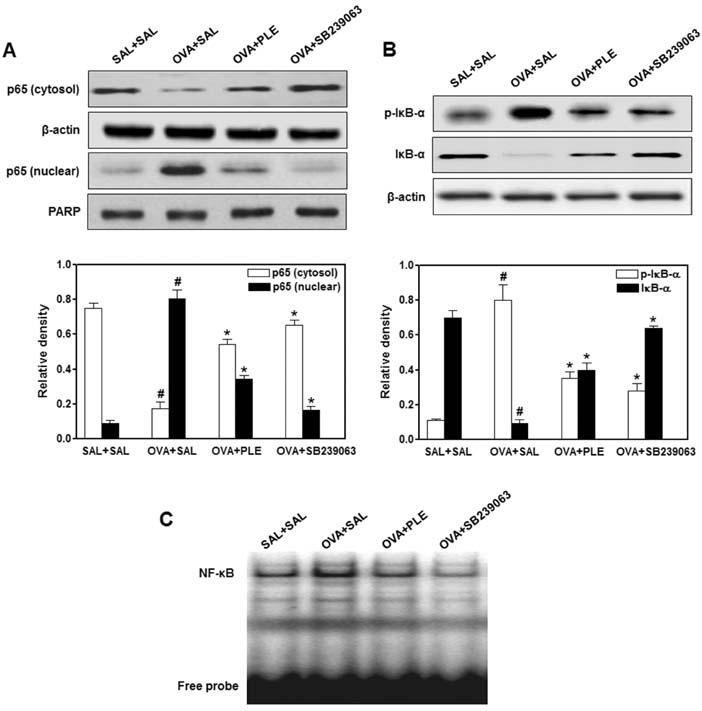
- Phellinus linteus has been used as a traditional herbal medicine in Asian countries and is known to have anti-tumor, immunomodulatory, anti-inflammatory, and anti-allergic activities. However, the protective effects of P. linteus against experimental asthma have not been fully investigated. The objective of this study was to determine whether P. linteus ethanol extract (PLE) suppresses inflammatory response in an OVA-induced asthma model. As expected, the oral administration of PLE significantly inhibited eosinophilic airway inflammation and airway hyperresponsiveness in OVA-challenged BALB/c mice. Supporting these data, the augmentation of Th2 cytokines (IL-4, IL-5, and IL-13), eotaxin, and adhesion molecules in lung tissues and bronchoalveolar lavage fluid after OVA inhalation was markedly attenuated by PLE. Furthermore, PLE reduced OVA-induced activation of NF-kappaB and p38 MAPK in lung tissues. Therefore, our results suggest the potential of P. linteus as a therapeutic agent for asthma.
Keyword
MeSH Terms
-
Administration, Oral
Animals
Asian Continental Ancestry Group
Asthma*
Bronchoalveolar Lavage Fluid
Cytokines
Eosinophils
Ethanol
Herbal Medicine
Humans
Inflammation
Inhalation
Interleukin-5
Lung
Mice*
NF-kappa B*
Ovum
p38 Mitogen-Activated Protein Kinases*
Cytokines
Ethanol
Interleukin-5
NF-kappa B
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454.
Article2. Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol. 2008; 26:205–232.
Article3. Imanifooladi AA, Yazdani S, Nourani MR. The role of nuclear factor-kappaB in inflammatory lung disease. Inflamm Allergy Drug Targets. 2010; 9:197–205.4. Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther. 2013; 140:290–305.
Article5. Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-kappaB inhibits established asthmatic reaction in mice. Int Immunopharmacol. 2004; 4:1817–1828.
Article6. Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013; 13:679–692.
Article7. Duan W, Chan JH, McKay K, Crosby JR, Choo HH, Leung BP, Karras JG, Wong WS. Inhaled p38alpha mitogen-activated protein kinase antisense oligonucleotide attenuates asthma in mice. Am J Respir Crit Care Med. 2005; 171:571–578.
Article8. Uyanoglu M, Canbek M, van Griensven LJ, Yamac M, Senturk H, Kartkaya K, Oglakci A, Turgak O, Kanbak G. Effects of polysaccharide from fruiting bodies of Agaricus bisporus, Agaricus brasiliensis, and Phellinus linteus on alcoholic liver injury. Int J Food Sci Nutr. 2014; in press: http://informahealthcare.com/doi/abs/10.3109/09637486.2013.869796.9. Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID, Yang KH, Kim HM. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology. 1999; 41:157–164.
Article10. Song M, Park DK, Park HJ. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J Ethnopharmacol. 2014; in press: http://dx.doi.org/10.1016/j.jep.2013.12.059.
Article11. Choi YH, Yan GH, Chai OH, Lim JM, Sung SY, Zhang X, Kim JH, Choi SH, Lee MS, Han EH, Kim HT, Song CH. Inhibition of anaphylaxis-like reaction and mast cell activation by water extract from the fruiting body of Phellinus linteus. Biol Pharm Bull. 2006; 29:1360–1365.
Article12. Lim BO, Yamada K, Cho BG, Jeon T, Hwang SG, Park T, Kang SA, Park DK. Comparative study on the modulation of IgE and cytokine production by Phellinus linteus grown on germinated brown Rice, Phellinus Linteus and germinated brown rice in murine splenocytes. Biosci Biotechnol Biochem. 2004; 68:2391–2394.
Article13. Hwang JS, Kwon HK, Kim JE, Rho J, Im SH. Immunomodulatory effect of water soluble extract separated from mycelium of Phellinus linteus on experimental atopic dermatitis. BMC Complement Altern Med. 2012; 12:159.
Article14. Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, Choe YH, Hong SH, Han HJ, Lee YR, Kim JS, Atlas D, Lee YC. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007; 39:756–768.
Article15. Boyce JA, Bochner B, Finkelman FD, Rothenberg ME. Advances in mechanisms of asthma, allergy, and immunology in 2011. J Allergy Clin Immunol. 2012; 129:335–341.
Article16. Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003; 24:640–647.
Article17. Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep. 2007; 7:18–26.
Article18. Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001; 1:108–116.
Article19. Mattes J, Foster PS. Regulation of eosinophil migration and Th2 cell function by IL-5 and eotaxin. Curr Drug Targets Inflamm Allergy. 2003; 2:169–174.
Article20. Li L, Xia Y, Nguyen A, Lai YH, Feng L, Mosmann TR, Lo D. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol. 1999; 162:2477–2487.21. Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. 2013; 4:263.
Article22. Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006; 118:551–559. quiz 560-561.
Article23. Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000; 105:651–663.
Article24. Vargaftig BB, Singer M. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am J Respir Cell Mol Biol. 2003; 28:410–419.
Article25. Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008; 121:5–10. quiz 11-12.26. Mayr SI, Zuberi RI, Zhang M, de Sousa-Hitzler J, Ngo K, Kuwabara Y, Yu L, Fung-Leung WP, Liu FT. IgE-dependent mast cell activation potentiates airway responses in murine asthma models. J Immunol. 2002; 169:2061–2068.
Article27. Kim SR, Lee KS, Park SJ, Min KH, Lee MH, Lee KA, Bartov O, Atlas D, Lee YC. A novel dithiol amide CB3 attenuates allergic airway disease through negative regulation of p38 mitogen-activated protein kinase. Am J Respir Crit Care Med. 2011; 183:1015–1024.
Article28. Chen YS, Lee SM, Lin CC, Liu CY. Hispolon decreases melanin production and induces apoptosis in melanoma cells through the downregulation of tyrosinase and microphthalmia-associated transcription factor (MITF) expressions and the activation of caspase-3, -8 and -9. Int J Mol Sci. 2014; 15:1201–1215.
Article29. Chen W, Feng L, Huang Z, Su H. Hispidin produced from Phellinus linteus protects against peroxynitrite-mediated DNA damage and hydroxyl radical generation. Chem Biol Interact. 2012; 199:137–142.
Article30. Huang GJ, Huang SS, Deng JS. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-kappaB, and MAPK activation in vitro and in vivo. PLoS One. 2012; 7:e35922.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- p38 MAPK and NF-kappaB are required for LPS-induced RANTES production in immortalized murine microglia (BV-2)
- NJK14047 Suppression of the p38 MAPK Ameliorates OVA-Induced Allergic Asthma during Sensitization and Challenge Periods
- Effect of intranasal rosiglitazone on airway inflammation and remodeling in a murine model of chronic asthma
- Anti-cancer Activities of Ginseng Extract Fermented with Phellinus linteus
- Lentinus edodes Suppresses Allergen-Induced Airway Hyperresponsiveness and Inflammation by Downregulating Nuclear Factor-kappa B Activity in a Murine Model of Allergic Asthma