Immune Netw.
2011 Oct;11(5):288-298. 10.4110/in.2011.11.5.288.
Role of Salvia miltiorrhiza for Modulation of Th2-derived Cytokines in the Resolution of Inflammation
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul 139-742, Korea. kimkj@syu.ac.kr
- 2College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea.
- KMID: 2168001
- DOI: http://doi.org/10.4110/in.2011.11.5.288
Abstract
- BACKGROUND
Salvia miltiorrhiza (SM) has been used to treat inflammatory diseases including edema and arthritis; however, the anti-inflammatory mechanism of SM action remains unresolved.
METHODS
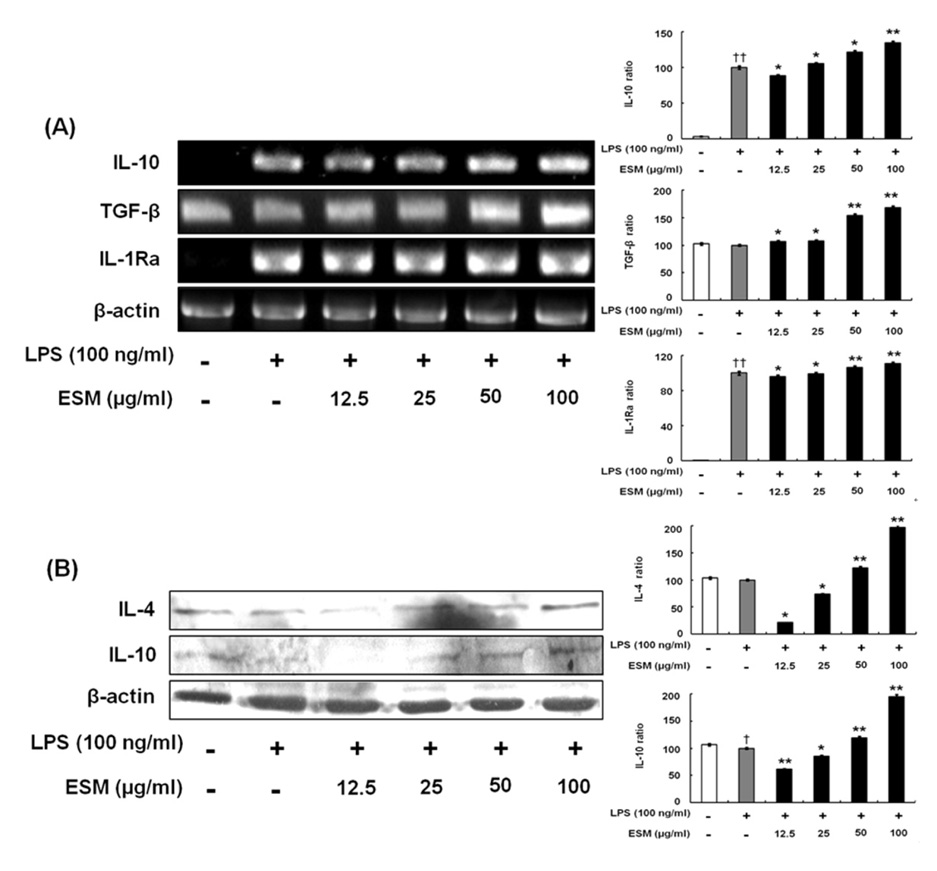
The effects of an ethanol extract of SM (ESM) on pro-inflammatory cytokines such as TNF-alpha, IL-1beta, IL-6, and NO, on anti-inflammatory cytokines including IL-4, IL-10, TGF-beta, and IL-1Ra have been studied in an attempt to elucidate the anti-inflammatory mechanism in murine macrophages.
RESULTS
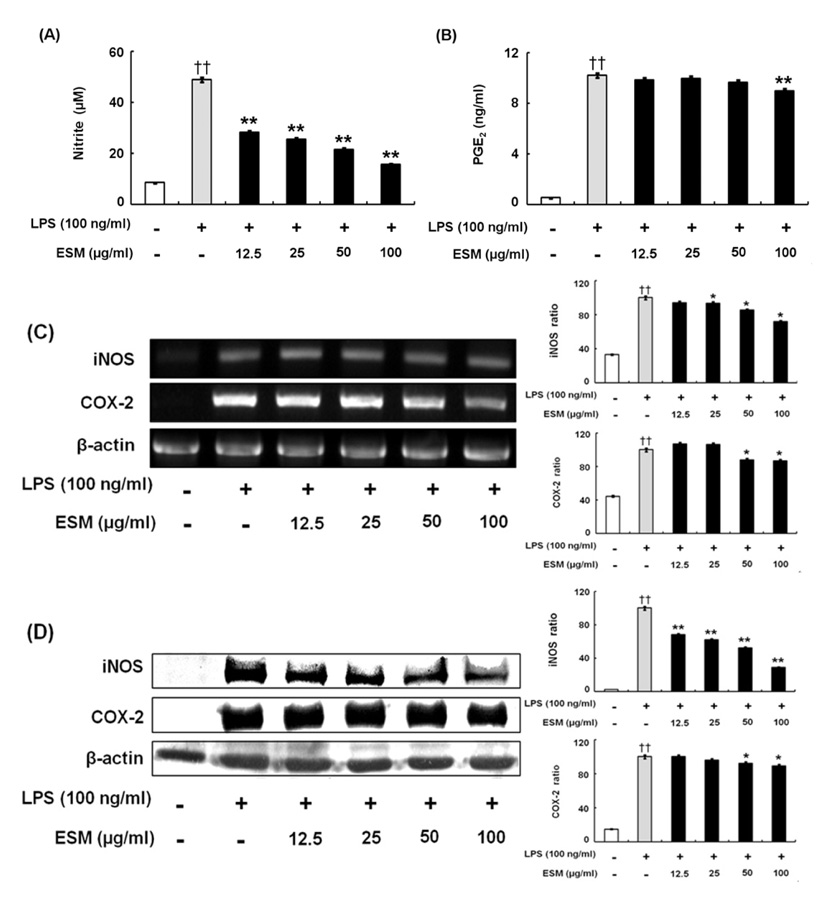
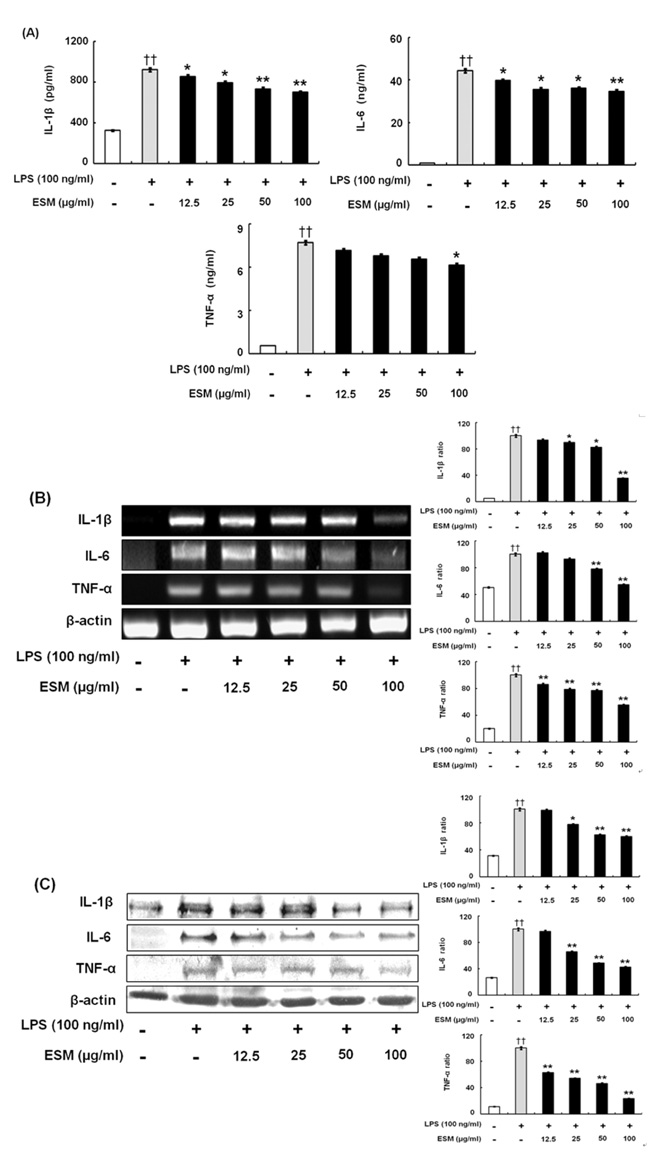
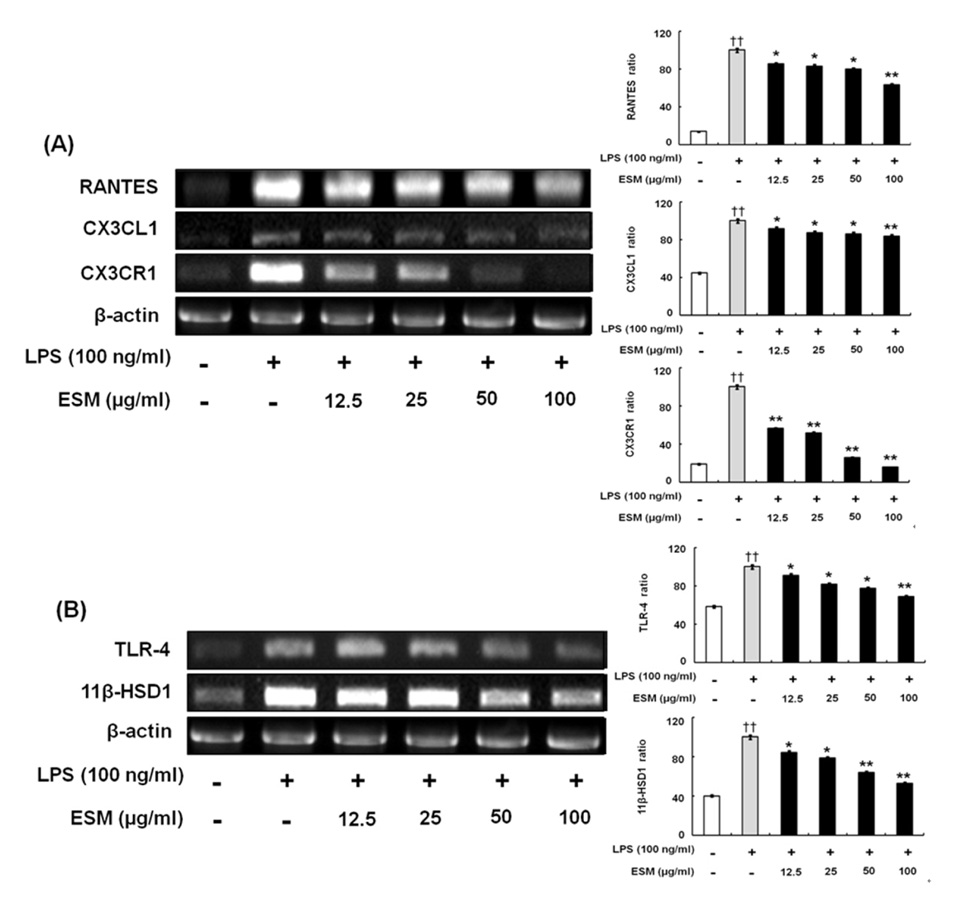
ESM inhibited the production of pro-inflammatory cytokines via down-regulation of gene and protein expression whereas it increased the anti-inflammatory cytokines. Furthermore, ESM inhibited the expression of the chemokines, RANTES and CX3CL1, as well as of inflammatory mediators such as TLR-4 and 11beta-HSD1.
CONCLUSION
These results indicated that the regulatory effects of ESM may be mediated though the suppression of pro-inflammatory cytokines as well as the induction of anti-inflammatory cytokines. Consequently, we speculate that ESM has therapeutic potential for inflammation-associated disorders.
MeSH Terms
-
Chemokine CCL5
Chemokines
Cytokines
Down-Regulation
Edema
Ethanol
Inflammation
Interleukin 1 Receptor Antagonist Protein
Interleukin-10
Interleukin-4
Interleukin-6
Salvia
Salvia miltiorrhiza
Transforming Growth Factor beta
Tumor Necrosis Factor-alpha
Chemokine CCL5
Chemokines
Cytokines
Ethanol
Interleukin 1 Receptor Antagonist Protein
Interleukin-10
Interleukin-4
Interleukin-6
Transforming Growth Factor beta
Tumor Necrosis Factor-alpha
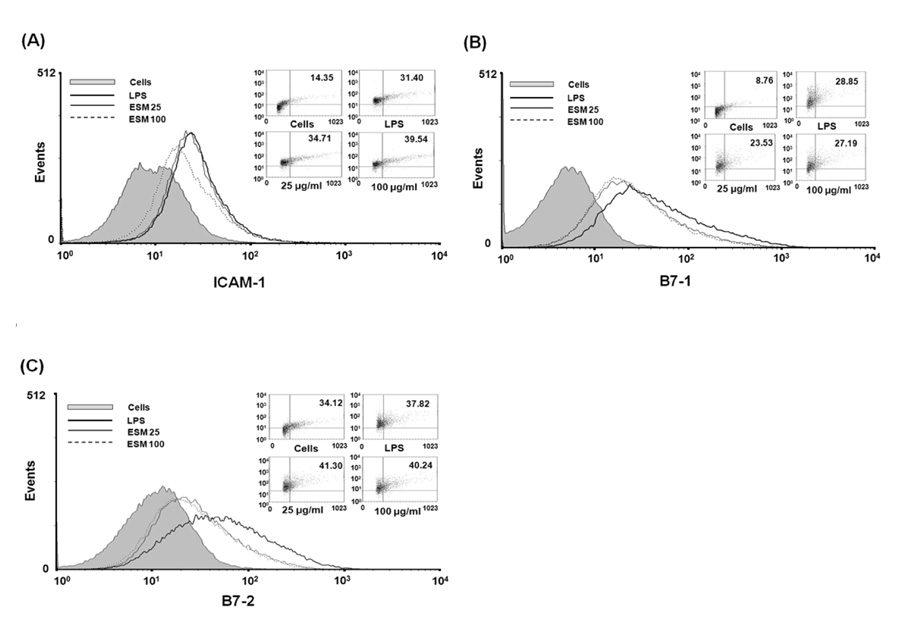
Figure
Reference
-
1. Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000. 117:1162–1172.
Article2. Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003. 169:203–214.
Article3. Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992. 103:264–274.
Article4. Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998. 11:1218–1221.
Article5. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006. 354:610–621.
Article6. Dinarello CA. Proinflammatory cytokines. Chest. 2000. 118:503–508.
Article7. Nair MP, Kandaswami C, Mahajan S, Chadha KC, Chawda R, Nair H, Kumar N, Nair RE, Schwartz SA. The flavonoid, quercetin, differentially regulates Th-1 (IFNgamma) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim Biophys Acta. 2002. 1593:29–36.
Article8. McKellar G, Madhok R, Singh G. The problem with NSAIDs: what data to believe? Curr Pain Headache Rep. 2007. 11:423–427.
Article9. Kim JK. Illustrated Natural Drugs Encyclopedia. 1989. Seoul: Namsandan Publishers.10. Dweck A. Kintzios SE, editor. The folklore and cosmetic use of various Salvia species. Sage The Genus Salvia. 2000. New York: Overseas Publishers;1–25.11. Duke J, Ayensu E. Medicinal Plants of China. 1985. Algonac, Mich: Reference Publications.12. Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin. 2000. 21:1089–1094.13. Ding M, Ye TX, Zhao GR, Yuan YJ, Guo ZX. Aqueous extract of Salvia miltiorrhiza attenuates increased endothelial permeability induced by tumor necrosis factor-alpha. Int Immunopharmacol. 2005. 5:1641–1651.
Article14. Chen TH, Hsu YT, Chen CH, Kao SH, Lee HM. Tanshinone IIA from Salvia miltiorrhiza induces heme oxygenase-1 expression and inhibits lipopolysaccharide-induced nitric oxide expression in RAW 264.7 cells. Mitochondrion. 2007. 7:101–105.
Article15. Jeon SJ, Son KH, Kim YS, Choi YH, Kim HP. Inhibition of prostaglandin and nitric oxide production in lipopolysaccharide-treated RAW 264.7 cells by tanshinones from the roots of Salvia miltiorrhiza bunge. Arch Pharm Res. 2008. 31:758–763.
Article16. Kim SY, Moon TC, Chang HW, Son KH, Kang SS, Kim HP. Effects of tanshinone I isolated from Salvia miltiorrhiza bunge on arachidonic acid metabolism and in vivo inflammatory responses. Phytother Res. 2002. 16:616–620.
Article17. Choi HS, Kim K. Tanshinones inhibit mast cell degranulation by interfering with IgE receptor-mediated tyrosine phosphorylation of PLCg2 and MAPK. Planta Med. 2004. 70:178–180.
Article18. Jang SI, Jeong SI, Kim KJ, Kim HJ, Yu HH, Park R, Kim HM, You YO. Tanshinone IIA from Salvia miltiorrhiza inhibits inducible nitric oxide synthase expression and production of TNF-, IL-1 and IL-6 in activated RAW 264.7 cells. Planta Med. 2003. 69:1057–1059.
Article19. Munoz C, Carlet J, Fitting C, Misset B, Blériot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991. 88:1747–1754.
Article20. Täuber MG, Moser B. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin Infect Dis. 1999. 28:1–11.
Article21. Hebert CA. Chemokines in disease:biology and clinical research. 1999. Totowa (NJ): Humana Press Inc.22. Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001. 22:83–87.
Article23. Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997. 385:640–644.
Article24. Matsunawa M, Isozaki T, Odai T, Yajima N, Takeuchi HT, Negishi M, Ide H, Adachi M, Kasama T. Increased serum levels of soluble fractalkine (CX3CL1) correlate with disease activity in rheumatoid vasculitis. Arthritis Rheum. 2006. 54:3408–3416.
Article25. Chen S, Bacon KB, Li L, Garcia GE, Xia Y, Lo D, Thompson DA, Siani MA, Yamamoto T, Harrison JK, Feng L. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J Exp Med. 1998. 188:193–198.
Article26. Schlöndorff D, Nelson PJ, Luckow B, Banas B. Chemokines and renal disease. Kidney Int. 1997. 51:610–621.
Article27. Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994. 6:865–873.
Article28. Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990. 136:1229–1233.29. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001. 344:907–916.
Article30. Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996. 17:64–102.
Article31. Akira S. Toll-like receptor signaling. J Biol Chem. 2003. 278:38105–38108.
Article32. Morikawa K, Zhang J, Nonaka M, Morikawa S. Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production. Int J Antimicrob Agents. 2002. 19:53–59.
Article33. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986. 136:2348–2357.34. Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997. 17:1–32.
Article35. Underhill DM, Bassetti M, Rudensky A, Aderem A. Dynamic interactions of macrophages with T cells during antigen presentation. J Exp Med. 1999. 190:1909–1914.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The anti-Influenza effect of a water soluble herbal extract from Salvia miltiorrhiza (Danshen) in vivo
- Cryptotanshinone but not tanshinone IIA inhibits angiogenesisin vitro
- 2-(4-Hydroxyphenyl)-5-(3-Hydroxypropenyl)-7-Methoxybenzofuran, a Novel Ailanthoidol Derivative, Exerts Anti-Inflammatory Effect through Downregulation of Mitogen-Activated Protein Kinase in Lipopolysaccharide-Treated RAW 264.7 Cells
- Combination of Probiotics and Salvia miltiorrhiza Polysaccharide Alleviates Hepatic Steatosis via Gut Microbiota Modulation and Insulin Resistance Improvement in High Fat-Induced NAFLD Mice
- Inhibition of α-Glucosidase by Abietane-Type Diterpenoids Isolated from Roots of Salvia miltiorrhiza