Diabetes Metab J.
2020 Apr;44(2):336-348. 10.4093/dmj.2019.0042.
Combination of Probiotics and Salvia miltiorrhiza Polysaccharide Alleviates Hepatic Steatosis via Gut Microbiota Modulation and Insulin Resistance Improvement in High Fat-Induced NAFLD Mice
- Affiliations
-
- 1Guangdong Provincial Key Laboratory of Gastroenterology, Department of Gastroenterology, Institute of Gastroenterology of Guangdong Province, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Gastroenterology, Sir Run Run Hospital, Nanjing Medical University, Nanjing, China
- KMID: 2502415
- DOI: http://doi.org/10.4093/dmj.2019.0042
Abstract
- Background
Nonalcoholic fatty liver disease (NAFLD) increases the risk of hepatocellular carcinoma, which is currently the leading cause of obesity-related cancer deaths in middle-aged men.
Methods
Probiotics with lipid-lowering function were screened from the fecal microbiota of healthy adults. Polysaccharide from different sources was screened for improving insulin resistance. The combination of probiotics and Salvia miltiorrhiza polysaccharide (LBM) was investigated for alleviating hepatic steatosis.
Results
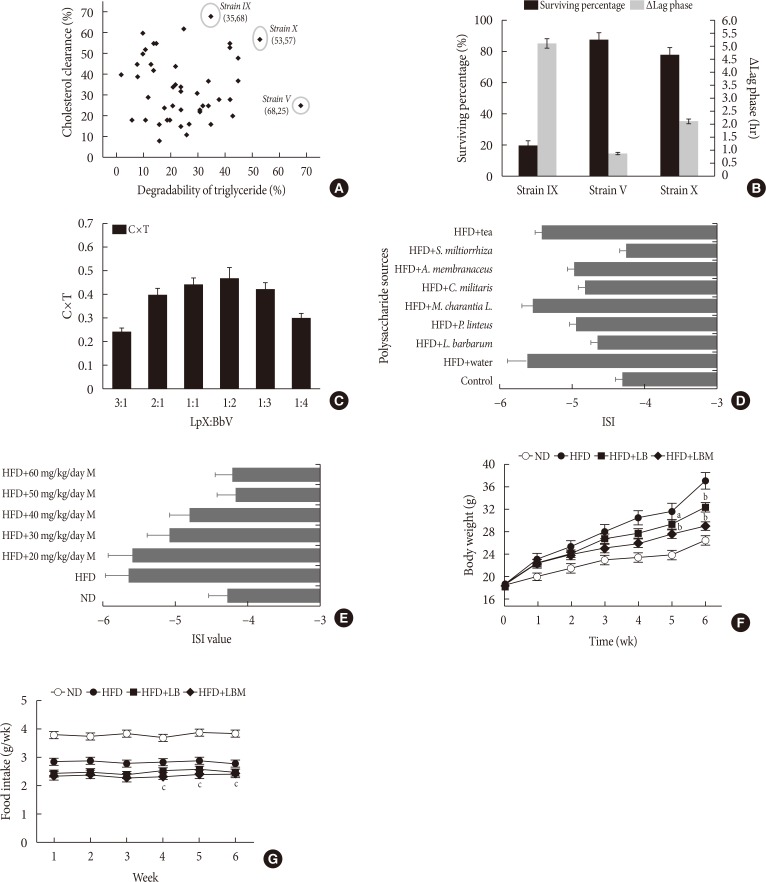
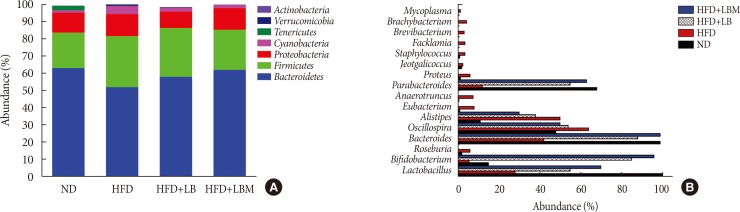
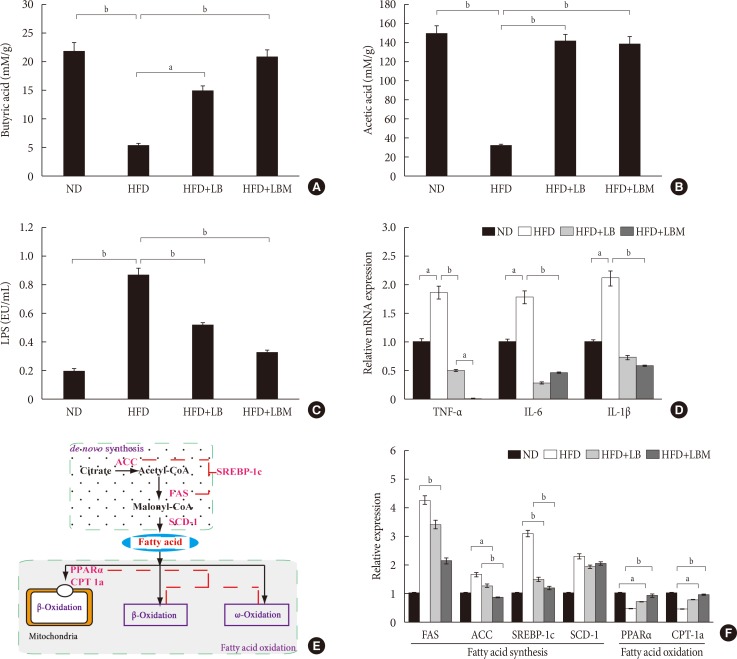
First, Bifidobacterium bifidum V (BbV) and Lactobacillus plantarum X (LpX) were obtained from the fecal microbiota of healthy adults. Second, to improve insulin resistance, a Salvia miltiorrhiza Bunge polysaccharide showing good performance in reducing insulin resistance was obtained. The liver total cholesterol (TC) and total triglyceride (TG) levels and the serum levels of free fatty acid, alanine transaminase, aspartate transaminase, low density lipoprotein cholesterol, TG, and TC can be significantly reduced through supplementation with LpX-BbV (LB) in NAFLD mice. Interestingly, the function of the probiotic LB can be enhanced by S. miltiorrhiza Bunge polysaccharide. Furthermore, the gut microbiota was modulated by LpX-BbV+S. miltiorrhiza Bunge polysaccharide (LBM). The lipopolysaccharide concentration of the LBM group was decreased by 73.6% compared to the NAFLD group. Ultimately, the mRNA concentrations of the proinflammatory cytokines (tumor necrosis factor α, interleukin 1β [IL-1β], and IL-6) decreased with LB and LBM treatment.
Conclusion
The results of this this study indicate that the LBM combination can be used as a therapeutic for ameliorating NAFLD via modulating the gut microbiota and improving insulin resistance.
Keyword
Figure
Reference
-
1. European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD). European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 2016; 9:65–90. PMID: 27055256.2. Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012; 23:203–208. PMID: 22129639.
Article3. Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010; 376:1916–1922. PMID: 21109302.
Article4. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013; 10:656–665. PMID: 24080776.
Article5. Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010; 7:691–701. PMID: 21045794.
Article6. Sanyal AJ. American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:1705–1725. PMID: 12404245.
Article7. Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015; 7:1450–1459. PMID: 26085906.
Article8. Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013; 5:1544–1560. PMID: 23666091.
Article9. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013; 10:686–690. PMID: 24042449.
Article10. Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000; 119:1340–1347. PMID: 11054393.
Article11. Kakino S, Ohki T, Nakayama H, Yuan X, Otabe S, Hashinaga T, Wada N, Kurita Y, Tanaka K, Hara K, Soejima E, Tajiri Y, Yamada K. Pivotal role of TNF-α in the development and progression of nonalcoholic fatty liver disease in a murine model. Horm Metab Res. 2018; 50:80–87. PMID: 28922680.
Article12. Bauer TM, Schwacha H, Steinbruckner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002; 97:2364–2370. PMID: 12358257.
Article13. Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001; 96:1251–1255. PMID: 11316178.
Article14. Thalheimer U, De Iorio F, Capra F, del Mar Lleo M, Zuliani V, Ghidini V, Tafi MC, Caburlotto G, Gennari M, Burroughs AK, Vantini I. Altered intestinal function precedes the appearance of bacterial DNA in serum and ascites in patients with cirrhosis: a pilot study. Eur J Gastroenterol Hepatol. 2010; 22:1228–1234. PMID: 20512041.
Article15. Kirsch R, Clarkson V, Verdonk RC, Marais AD, Shephard EG, Ryffel B, de la M Hall P. Rodent nutritional model of steatohepatitis: effects of endotoxin (lipopolysaccharide) and tumor necrosis factor alpha deficiency. J Gastroenterol Hepatol. 2006; 21:174–182. PMID: 16706830.
Article16. Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002; 35:367–372. PMID: 11826410.
Article17. Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010; 298:G851–G859. PMID: 20224007.
Article18. Xu RY, Wan YP, Fang QY, Lu W, Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr. 2012; 50:72–77. PMID: 22247604.
Article19. Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013; 19:6911–6918. PMID: 24187469.
Article20. Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, Galvano F. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012; 57:545–553. PMID: 21901256.
Article21. Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009; 15:1546–1558. PMID: 19442172.
Article22. Azat R, Liu Y, Li W, Kayir A, Lin DB, Zhou WW, Zheng XD. Probiotic properties of lactic acid bacteria isolated from traditionally fermented Xinjiang cheese. J Zhejiang Univ Sci B. 2016; 17:597–609. PMID: 27487805.
Article23. Mei L, Tang Y, Li M, Yang P, Liu Z, Yuan J, Zheng P. Co-Administration of cholesterol-lowering probiotics and anthraquinone from Cassia obtusifolia L. Ameliorate non-alcoholic fatty liver. PLoS One. 2015; 10:e0138078. PMID: 26375281.
Article24. Saravanan G, Ponmurugan P. Ameliorative potential of S-allylcysteine: effect on lipid profile and changes in tissue fatty acid composition in experimental diabetes. Exp Toxicol Pathol. 2012; 64:639–644. PMID: 21216577.
Article25. Zhang W, Zheng L, Zhang Z, Hai CX. Protective effect of a water-soluble polysaccharide from Salvia miltiorrhiza Bunge on insulin resistance in rats. Carbohydr Polym. 2012; 89:890–898. PMID: 24750877.
Article26. Xing XY, Li YF, Fu ZD, Chen YY, Wang YF, Liu XL, Liu WY, Li GW. Antihypertensive effect of metformin in essential hypertensive patients with hyperinsulinemia. Zhonghua Nei Ke Za Zhi. 2010; 49:14–18. PMID: 20356474.27. Zhang Y, Hu T, Zhou H, Zhang Y, Jin G, Yang Y. Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int J Biol Macromol. 2016; 83:126–132. PMID: 26627601.
Article28. Ren D, Hu Y, Luo Y, Yang X. Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct. 2015; 6:3342–3350. PMID: 26267675.
Article29. Bao Y, Zhang Y, Zhang Y, Liu Y, Wang S, Dong X, Wang Y, Zhang H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control. 2010; 21:695–701.
Article30. Huang Y, Wang X, Wang J, Wu F, Sui Y, Yang L, Wang Z. Lactobacillus plantarum strains as potential probiotic cultures with cholesterol-lowering activity. J Dairy Sci. 2013; 96:2746–2753. PMID: 23498020.
Article31. Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol. 2007; 45:2761–2764. PMID: 17626177.
Article32. Adler P, Frey LJ, Berger A, Bolten CJ, Hansen CE, Wittmann C. The key to acetate: metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions. Appl Environ Microbiol. 2014; 80:4702–4716. PMID: 24837393.
Article33. Kump PK, Grochenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, Deutschmann A, Wenzl HH, Petritsch W, Krejs GJ, Gorkiewicz G, Hogenauer C. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013; 19:2155–2165. PMID: 23899544.
Article34. Watts GF, Burke V. Lipid-lowering trials in the primary and secondary prevention of coronary heart disease: new evidence, implications and outstanding issues. Curr Opin Lipidol. 1996; 7:341–355. PMID: 9117137.
Article35. Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761. PMID: 16968800.36. Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014; 15:8591–8638. PMID: 24830559.
Article37. Ding W, Shi C, Chen M, Zhou J, Long R, Guo X. Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J Funct Foods. 2017; 32:324–332.
Article38. Ooi LG, Liong MT. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 2010; 11:2499–2522. PMID: 20640165.
Article39. Liu W, Xi X, Sudu Q, Kwok L, Guo Z, Hou Q, Menhe B, Sun T, Zhang H. High-throughput sequencing reveals microbial community diversity of Tibetan naturally fermented yak milk. Ann Microbiol. 2015; 65:1741–1751.
Article41. Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011; 53:994–1002. PMID: 22002980.
Article42. Farrell GC. Signalling links in the liver: knitting SOCS with fat and inflammation. J Hepatol. 2005; 43:193–196. PMID: 15913829.
Article43. Xu S, Dou Y, Ye B, Wu Q, Wang Y, Hu M, Ma F, Rong X, Guo J. Ganoderma lucidum polysaccharides improve insulin sensitivity by regulating inflammatory cytokines and gut microbiota composition in mice. J Funct Foods. 2017; 38(Pt A):545–552.
Article44. Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G1018–G1036. PMID: 27686615.
Article45. Durai P, Batool M, Choi S. Structure and effects of cyanobacterial lipopolysaccharides. Mar Drugs. 2015; 13:4217–4230. PMID: 26198237.
Article46. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006; 444:1022–1023. PMID: 17183309.47. Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008; 455:1109–1113. PMID: 18806780.
Article48. Marchesi JR, Holmes E, Khan F, Kochhar S, Scanlan P, Shanahan F, Wilson ID, Wang Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J Proteome Res. 2007; 6:546–551. PMID: 17269711.
Article49. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444:1027–1031. PMID: 17183312.
Article50. de Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, de Vogel-van den Bosch J, Kleerebezem M, Muller M, van der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012; 303:G589–G599. PMID: 22700822.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the Combination of Evogliptin and Leucine on Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice
- Modulation of Gut Microbiota: Potential Mechanism of Diabetes Remission after Bariatric/Metabolic Surgery
- Recent update on pathogenesis of nonalcoholic fatty liver disease
- Umbelliferone Ameliorates Hepatic Steatosis and Lipid-Induced ER Stress in High-Fat Diet-Induced Obese Mice
- Nonalcoholic fatty liver disease and insulin resistance in children