Clin Exp Vaccine Res.
2013 Jul;2(2):128-134. 10.7774/cevr.2013.2.2.128.
Intranasal immunization with a flagellin-adjuvanted peptide anticancer vaccine prevents tumor development by enhancing specific cytotoxic T lymphocyte response in a mouse model
- Affiliations
-
- 1Clinical Vaccine R&D Center, Chonnam National University, Gwangju, Korea. selee@chonnam.ac.kr
- 2Department of Pharmacology and Dental Therapeutics, School of Dentistry, Chonnam National University, Gwangju, Korea.
- 3Department of Microbiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2167723
- DOI: http://doi.org/10.7774/cevr.2013.2.2.128
Abstract
- PURPOSE
Human papillomavirus (HPV) is a significant cause of cervical cancer-related deaths worldwide. Because HPV is a sexually transmitted mucosal pathogen, enhancement of antigen-specific mucosal immune response likely serves good strategy for vaccination. However, mucosal vaccines generally do not induce strong enough immune responses. Previously we proved that a bacterial flagellin, Vibrio vulnificus FlaB, induce strong antigen-specific immune responses by stimulating the Toll-like receptor 5. In this study, we tested whether FlaB could serve as an effective mucosal adjuvant for a peptide-based HPV preventive cancer vaccine.
MATERIALS AND METHODS
Mice were intranasally administered with a mixture of FlaB and E6/E7 protective peptides in 5-day interval for a total of two times. Five-days after the last vaccination, cellular immune responses of the vaccinated mice were analyzed. Tumor growth was also observed after a subcutaneous implantation of TC-1 cells bearing E6/E7 antigens.
RESULTS
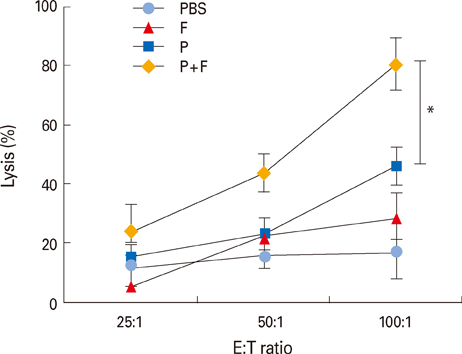
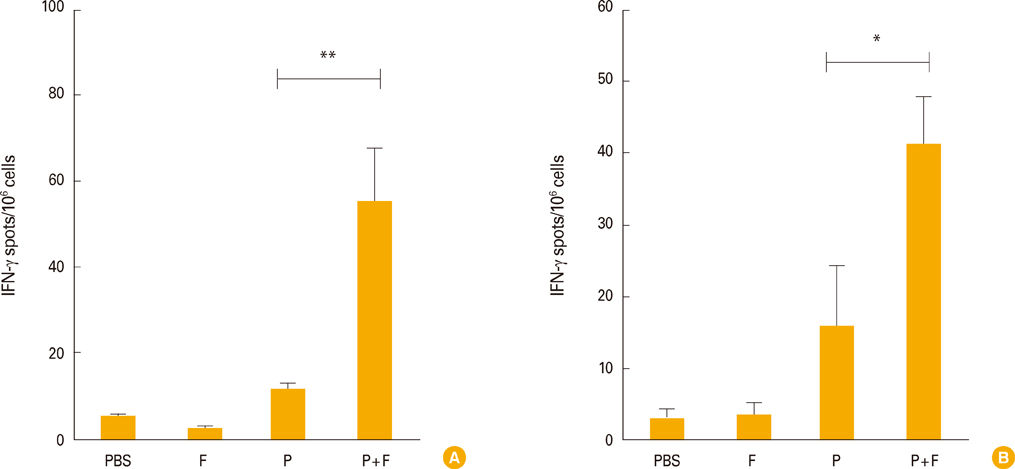
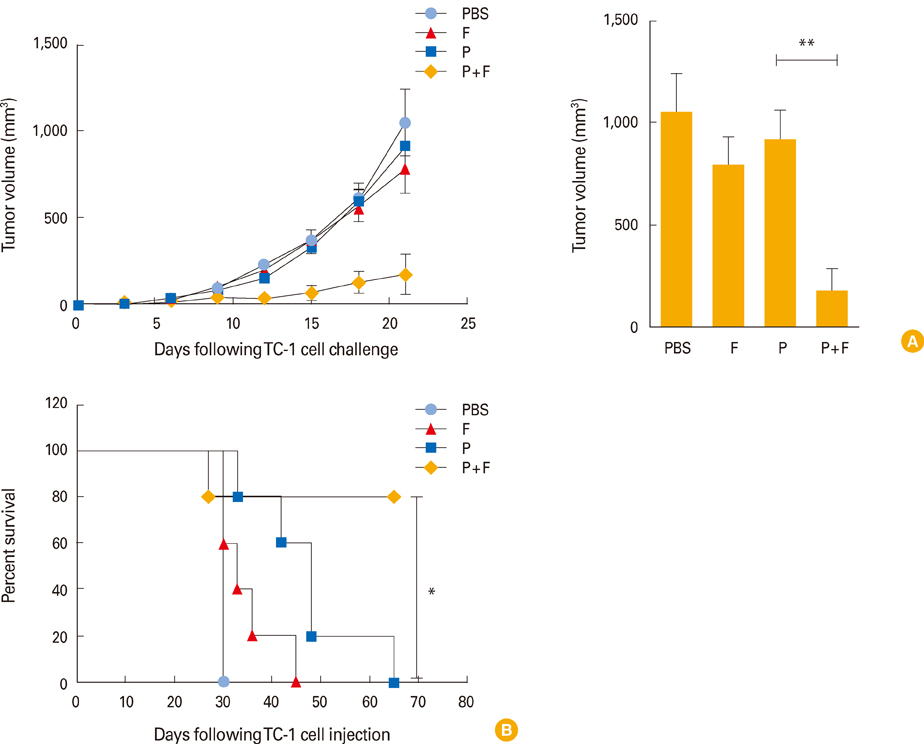
Intranasal administration of the E6/E7 peptide mixture with FlaB elicited a strong antigen-specific cytotoxic T lymphocyte activity and antigen-specific interferon-gamma production from splenocytes and cervical lymph node cells. Furthermore, FlaB, as a mucosal adjuvant, conferred an excellent protection against TC-1 tumor challenge with high survival rates in E6/E7 immunized animals.
CONCLUSION
These results indicate that FlaB can be a promising mucosal adjuvant for nasal HPV vaccine development.
MeSH Terms
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
Article2. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189:12–19.
Article3. Kim D, Gambhira R, Karanam B, et al. Generation and characterization of a preventive and therapeutic HPV DNA vaccine. Vaccine. 2008; 26:351–360.
Article4. Lin K, Doolan K, Hung CF, Wu TC. Perspectives for preventive and therapeutic HPV vaccines. J Formos Med Assoc. 2010; 109:4–24.
Article5. Roden RB, Ling M, Wu TC. Vaccination to prevent and treat cervical cancer. Hum Pathol. 2004; 35:971–982.
Article6. Mahdavi A, Monk BJ. Vaccines against human papillomavirus and cervical cancer: promises and challenges. Oncologist. 2005; 10:528–538.
Article7. Sin JI, Kim JM, Bae SH, Lee IH, Park JS, Ryoo HM. Adoptive transfer of human papillomavirus E7-specific CTL enhances tumor chemoresponse through the perforin/granzyme-mediated pathway. Mol Ther. 2009; 17:906–913.
Article8. Manuri PR, Nehete B, Nehete PN, et al. Intranasal immunization with synthetic peptides corresponding to the E6 and E7 oncoproteins of human papillomavirus type 16 induces systemic and mucosal cellular immune responses and tumor protection. Vaccine. 2007; 25:3302–3310.
Article9. Zwaveling S, Ferreira Mota SC, Nouta J, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002; 169:350–358.
Article10. Vambutas A, DeVoti J, Nouri M, et al. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005; 23:5271–5280.
Article11. Sharma RK, Elpek KG, Yolcu ES, et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res. 2009; 69:4319–4326.
Article12. Lee SE, Kim SY, Jeong BC, et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006; 74:694–702.
Article13. Nguyen CT, Kim SY, Kim MS, Lee SE, Rhee JH. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine. 2011; 29:5731–5739.
Article14. Hong SH, Byun YH, Nguyen CT, et al. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine. 2012; 30:466–474.
Article15. Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res. 2012; 1:50–63.
Article16. Feltkamp MC, Smits HL, Vierboom MP, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993; 23:2242–2249.
Article17. Seo SH, Kim KS, Park SH, et al. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 2011; 18:488–495.
Article18. Yu YA, Shabahang S, Timiryasova TM, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004; 22:313–320.
Article19. Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res. 2007; 13:341–349.
Article20. Sin JI. MyD88 signal is required for more efficient induction of Ag-specific adaptive immune responses and antitumor resistance in a human papillomavirus E7 DNA vaccine model. Vaccine. 2011; 29:4125–4131.
Article21. Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001; 410:1099–1103.
Article22. Ramos HC, Rumbo M, Sirard JC. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 2004; 12:509–517.
Article23. Seo SH, Jin HT, Park SH, Youn JI, Sung YC. Optimal induction of HPV DNA vaccine-induced CD8+ T cell responses and therapeutic antitumor effect by antigen engineering and electroporation. Vaccine. 2009; 27:5906–5912.
Article24. Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005; 175:8051–8059.
Article25. Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology. 2008; 135:518–528.
Article26. Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011; 71:2466–2475.
Article27. Sfondrini L, Rossini A, Besusso D, et al. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J Immunol. 2006; 176:6624–6630.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Antimicrobial Peptide LL-37 as an Adjuvant for Middle East Respiratory Syndrome-Coronavirus Antigen Induces an Efficient Protective Immune Response Against Viral Infection After Intranasal Immunization
- Enhanced CEA-specific Immune Responses by Tat-LLO Fusion Protein
- Comparative evaluation of gold nanoparticles and Alum as immune enhancers against rabies vaccine and related immune reactivity, physiological, and histopathological alterations: in vivo study
- Effect of the Heat Shock Protein 70 on the Adjuvanticity Induced by a Bacterial Flagellin of Vibrio ulnificus
- Efficient amplification of melanoma-specific CD8+ T cells using artificial antigen presenting complex