Healthc Inform Res.
2016 Apr;22(2):120-128. 10.4258/hir.2016.22.2.120.
Functional Profiling of Human MeCP2 by Automated Data Comparison Analysis and Computerized Expression Pathway Modeling
- Affiliations
-
- 1Department of Emergency Medical Technology, Dong-Eui Institute of Technology, Busan, Korea.
- 2Department of Biological Sciences, Inha University, Incheon, Korea.
- 3Department of Emergency Medicine, Dong-A University College of Medicine, Busan, Korea.
- 4Codes Division, Insilicogen Inc., Suwon, Korea.
- 5Department of Molecular Biology, Pusan National University, Busan, Korea.
- 6Supercomputing Center, Pusan National University, Busan, Korea. kimcm@pusan.ac.kr
- 7Research Center for Anti-Aging Technology Development, Pusan National University, Busan, Korea.
- 8Department of Medical Informatics, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 2166950
- DOI: http://doi.org/10.4258/hir.2016.22.2.120
Abstract
OBJECTIVES
Methyl-CpG binding protein 2 (MeCP2) is a ubiquitous epigenetic factor that represses gene expression by modifying chromatin. Mutations in the MeCP2 gene cause Rett syndrome, a progressive neurodevelopmental disorder. Recent studies also have shown that MeCP2 plays a role in carcinogenesis. Specifically, functional ablation of MeCP2 suppresses cell growth and leads to the proliferation of cancer cells. However, MeCP2's function in adult tissues remains poorly understood. We utilized a weight matrix-based comparison software to identify transcription factor binding site (TFBS) of MeCP2-regulated genes, which were recognized by cDNA microarray analysis.
METHODS
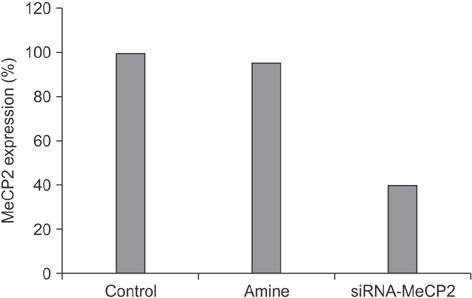
MeCP2 expression was silenced using annealed siRNA in HEK293 cells, and then a cDNA microarray analysis was performed. Functional analysis was carried out, and transcriptional levels in target genes regulated by MeCP2 were investigated. TFBS analysis was done within genes selected by the cDNA microarray analysis, using a weight matrix-based program and the TRANSFAC 6.0 database.
RESULTS
Among the differentially expressed genes with a change in expression greater than two-fold, 189 genes were up-regulated and 91 genes were down-regulated. Genes related to apoptosis and cell proliferation (JUN, FOSL2, CYR61, SKIL, ATF3, BMABI, BMPR2, RERE, and FALZ) were highly up-regulated. Genes with anti-apoptotic and anti-proliferative functions (HNRPA0, HIS1, and FOXC1) were down-regulated. Using TFBS analysis within putative promoters of novel candidate target genes of MeCP2, disease-related transcription factors were identified.
CONCLUSIONS
The present results provide insights into the new target genes regulated by MeCP2 under epigenetic control. This information will be valuable for further studies aimed at clarifying the pathogenesis of Rett syndrome and neoplastic diseases.
Keyword
MeSH Terms
-
Adult
Apoptosis
Binding Sites
Carcinogenesis
Carrier Proteins
Cell Proliferation
Chromatin
Epigenomics
Gene Expression
HEK293 Cells
Humans*
Methyl-CpG-Binding Protein 2
Microarray Analysis
Oligonucleotide Array Sequence Analysis
Rett Syndrome
RNA, Small Interfering
Transcription Factors
Carrier Proteins
Chromatin
Methyl-CpG-Binding Protein 2
RNA, Small Interfering
Transcription Factors
Figure
Reference
-
1. Rett A. Uber ein eigenartiges hirnatrophisches Syndrom bei Hyperammonamie im Kindesalter. Wien Med Wochenschr. 1966; 116(37):723–726.2. Hoffbuhr K, Devaney JM, LaFleur B, Sirianni N, Scacheri C, Giron J, et al. MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology. 2001; 56(11):1486–1495.
Article3. Imessaoudene B, Bonnefont JP, Royer G, Cormier-Daire V, Lyonnet S, Lyon G, et al. MECP2 mutation in nonfatal, non-progressive encephalopathy in a male. J Med Genet. 2001; 38(3):171–174.
Article4. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003; 33:Suppl. 245–254.
Article5. Bernard D, Gil J, Dumont P, Rizzo S, Monte D, Quatannens B, et al. The methyl-CpG-binding protein MECP2 is required for prostate cancer cell growth. Oncogene. 2006; 25(9):1358–1366.
Article6. Billard LM, Magdinier F, Lenoir GM, Frappart L, Dante R. MeCP2 and MBD2 expression during normal and pathological growth of the human mammary gland. Oncogene. 2002; 21(17):2704–2712.
Article7. Darwanto A, Kitazawa R, Maeda S, Kitazawa S. MeCP2 and promoter methylation cooperatively regulate E-cadherin gene expression in colorectal carcinoma. Cancer Sci. 2003; 94(5):442–447.
Article8. Hite KC, Adams VH, Hansen JC. Recent advances in MeCP2 structure and function. Biochem Cell Biol. 2009; 87(1):219–227.9. Wingender E. TRANSFAC, TRANSPATH and CYTOMER as starting points for an ontology of regulatory networks. In Silico Biol. 2004; 4(1):55–61.10. Wingender E, Chen X, Fricke E, Geffers R, Hehl R, Liebich I, et al. The TRANSFAC system on gene expression regulation. Nucleic Acids Res. 2001; 29(1):281–283.
Article11. Kel AE, Gossling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, Wingender E. MATCH: a tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res. 2003; 31(13):3576–3579.
Article12. Pairo E, Maynou J, Marco S, Perera A. A subspace method for the detection of transcription factor binding sites. Bioinformatics. 2012; 28(10):1328–1335.
Article13. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001; 98(9):5116–5121.
Article14. Hashimoto Y, Akiyama Y, Yuasa Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS One. 2013; 8(5):e62589.
Article15. Hill DP, Adams N, Bada M, Batchelor C, Berardini TZ, Dietze H, et al. Dovetailing biology and chemistry: integrating the Gene Ontology with the ChEBI chemical ontology. BMC Genomics. 2013; 14:513.
Article16. Zhao LY, Zhang J, Guo B, Yang J, Han J, Zhao XG, et al. MECP2 promotes cell proliferation by activating ERK1/2 and inhibiting p38 activity in human hepatocellular carcinoma HEPG2 cells. Cell Mol Biol (Noisy-le-grand). 2013; Suppl 59. OL1876–OL1881.17. Swerdlow RH. Mitochondrial DNA: related mitochondrial dysfunction in neurodegenerative diseases. Arch Pathol Lab Med. 2002; 126(3):271–280.
Article18. Nuber UA, Kriaucionis S, Roloff TC, Guy J, Selfridge J, Steinhoff C, et al. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum Mol Genet. 2005; 14(15):2247–2256.
Article19. Squillaro T, Alessio N, Cipollaro M, Renieri A, Giordano A, Galderisi U. Partial silencing of methyl cytosine protein binding 2 (MECP2) in mesenchymal stem cells induces senescence with an increase in damaged DNA. FASEB J. 2010; 24(5):1593–1603.
Article20. Rasti M, Arabsolghar R, Khatooni Z, Mostafavi-Pour Z. p53 Binds to estrogen receptor 1 promoter in human breast cancer cells. Pathol Oncol Res. 2012; 18(2):169–175.
Article21. Yang LH, Han Y, Li G, Xu HT, Jiang GY, Miao Y, et al. Axin gene methylation status correlates with radiosensitivity of lung cancer cells. BMC Cancer. 2013; 13:368.
Article22. Xu X, Jin H, Liu Y, Liu L, Wu Q, Guo Y, et al. The expression patterns and correlations of claudin-6, methy-CpG binding protein 2, DNA methyltransferase 1, histone deacetylase 1, acetyl-histone H3 and acetyl-histone H4 and their clinicopathological significance in breast invasive ductal carcinomas. Diagn Pathol. 2012; 7:33.
Article23. Bowser R, Reilly S. Expression of FAC1 in activated microglia during Alzheimer's disease. Neurosci Lett. 1998; 253(3):163–166.
Article24. Yanagisawa H, Bundo M, Miyashita T, Okamura-Oho Y, Tadokoro K, Tokunaga K, et al. Protein binding of a DRPLA family through arginine-glutamic aciddipeptide repeats is enhanced by extended polyglutamine. Hum Mol Genet. 2000; 9(9):1433–1442.
Article25. Joulie M, Miotto B, Defossez PA. Mammalian methylbinding proteins: what might they do? Bioessays. 2010; 32(12):1025–1032.
Article26. Chen Y, Luo J, Tian R, Sun H, Zou S. miR-373 negatively regulates methyl-CpG-binding domain protein 2 (MBD2) in hilar cholangiocarcinoma. Dig Dis Sci. 2011; 56(6):1693–1701.
Article27. Yan Z, Xiong Y, Xu W, Li M, Cheng Y, Chen F, et al. Identification of recurrence-related genes by integrating microRNA and gene expression profiling of gastric cancer. Int J Oncol. 2012; 41(6):2166–2174.
Article28. Jolly ER, Chin CS, Herskowitz I, Li H. Genome-wide identification of the regulatory targets of a transcription factor using biochemical characterization and computational genomic analysis. BMC Bioinformatics. 2005; 6:275.
Article29. Bernard B, Thorsson V, Rovira H, Shmulevich I. Increasing coverage of transcription factor position weight matrices through domain-level homology. PLoS One. 2012; 7(8):e42779.
Article30. Sapkota Y, Robson P, Lai R, Cass CE, Mackey JR, Damaraju S. A two-stage association study identifies methyl-CpG-binding domain protein 2 gene polymorphisms as candidates for breast cancer susceptibility. Eur J Hum Genet. 2012; 20(6):682–689.
Article31. Ping SY, Shen KH, Yu DS. Epigenetic regulation of vascular endothelial growth factor a dynamic expression in transitional cell carcinoma. Mol Carcinog. 2013; 52(7):568–579.
Article32. Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005; 14(4):483–492.
Article33. Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent BDNF transcription, dendritic growth, and spine maturation. Neuron. 2006; 52(2):255–269.
Article34. Babbio F, Castiglioni I, Cassina C, Gariboldi MB, Pistore C, Magnani E, et al. Knock-down of methyl CpG-binding protein 2 (MeCP2) causes alterations in cell proliferation and nuclear lamins expression in mammalian cells. BMC Cell Biol. 2012; 13:19.
Article35. van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Amarouchi K, et al. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol Psychiatry. 2012; 71(4):309–316.
Article36. Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998; 393(6683):386–389.
Article37. Wang JT, Wu TT, Bai L, Ding L, Hao M, Wang Y. Effect of folate in modulating the expression of DNA methyltransferase 1 and methyl-CpG-binding protein 2 in cervical cancer cell lines. Zhonghua Liu Xing Bing Xue Za Zhi. 2013; 34(2):173–177.38. Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004; 429(6990):457–463.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on Development of the Automated Critical Pathway for Patients with Cesarean Section
- Hyperoxygenation Treatment Reduces Beta-amyloid Deposition via MeCP2-dependent Upregulation of MMP-2 and MMP-9 in the Hippocampus of Tg-APP/PS1 Mice
- Claustral MeCP2 Regulates Methamphetamine-induced Conditioned Place Preference in Cynomolgus Monkey
- Comparison of oligonucleotide-microarray and serial analysis of gene expression (SAGE) in transcript profiling analysis of megakaryocytes derived from CD34+ cells
- HisCoM-PAGE: software for hierarchical structural component models for pathway analysis of gene expression data