Clin Exp Otorhinolaryngol.
2011 Jun;4(2):77-82.
Protective Effect of Minocycline Against Cisplatin-induced Ototoxicity
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Cheonan Hospital, Soonchunhyang University School of Medicine, Cheonan, Korea.
- 2Medical Laser Research Center, Dankook University, Cheonan, Korea.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. yscho@skku.edu
Abstract
OBJECTIVES
Cisplatin, a widely used chemotherapeutic agent, has serious side effects, including nephrotoxicity and ototoxicity. Minocycline is a semisynthetic second-generation tetracycline that exerts anti-inflammatory and neuroprotective effects. The purpose of this study was to elucidate the protective effect of minocycline against cisplatin-induced ototoxicity in the auditory hair cell.
METHODS
The House Ear Institute-Organ of Corti 1 (HEI-OC1) cell line and guinea pigs were used for in vitro and in vivo experiments. Cells were exposed to cisplatin with or without pre-treatment with minocycline. Cell survival was analyzed using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide). Whole-cell lysates were collected and immunoblotted with antibodies against Bcl-2, p-c-Jun, active caspase-3, cleaved poly (ADP-ribose) polymerase (PARP), and apoptosis-inducing factor (AIF). The guinea pigs received intraperitoneal injections of cisplatin alone or following minocycline pretreatment. The auditory brainstem response was tested and the cochleae were harvested and evaluated using scanning electron microscopy.
RESULTS
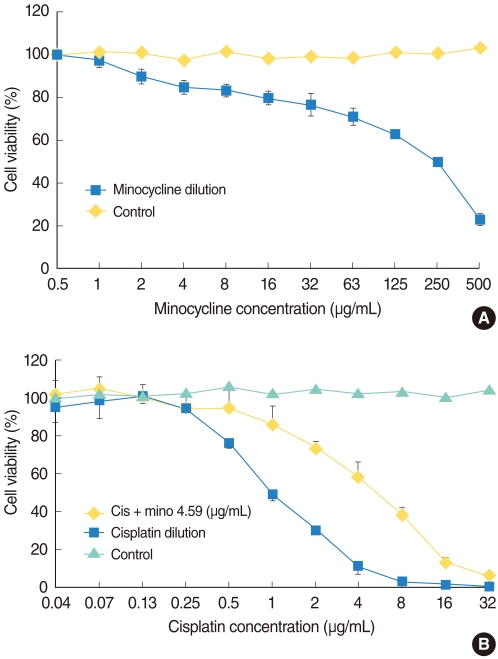
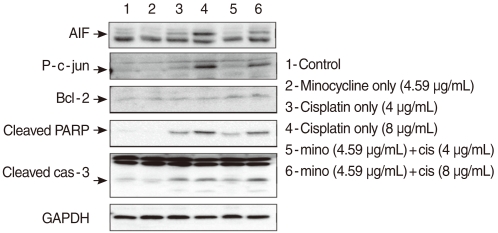
Survival significantly increased in cells pretreated with minocycline compared with cells exposed to cisplatin alone. Cisplatin treatment increased the expression of active caspase 3, p-c Jun, PARP, and AIF, and pretreatment with minocycline attenuated this response. In animal study, the threshold shift by cisplatin injection in the auditory brainstem response was less pronounced in animals pretreated with minocycline. Scanning electron microscopy revealed more severe damage to the outer hair cells at the basal and middle turns than the apical turn.
CONCLUSION
Minocycline partially protects against cisplatin-induced ototoxicity via both caspase-dependent and independent apoptosis pathways.
Keyword
MeSH Terms
-
Animals
Antibodies
Apoptosis
Apoptosis Inducing Factor
Caspase 3
Cell Line
Cell Survival
Cisplatin
Cochlea
Ear
Electrons
Evoked Potentials, Auditory, Brain Stem
Guinea Pigs
Hair
Injections, Intraperitoneal
Microscopy, Electron, Scanning
Minocycline
Neuroprotective Agents
Tetracycline
Antibodies
Apoptosis Inducing Factor
Caspase 3
Cisplatin
Minocycline
Neuroprotective Agents
Tetracycline
Figure
Reference
-
1. Hamers FP, Klis SF, Gispen WH, Smoorenburg GF. Application of a neuroprotective ACTH(4-9) analog to affect cisplatin ototoxicity: an electrocochleographic study in guinea pigs. Eur Arch Otorhinolaryngol. 1994; 251(1):23–29. PMID: 8179863.
Article2. Ravi R, Somani SM, Rybak LP. Mechanism of cisplatin ototoxicity: antioxidant system. Pharmacol Toxicol. 1995; 6. 76(6):386–394. PMID: 7479581.
Article3. Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001; 12. 12(12):2683–2690. PMID: 11729237.
Article4. Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level: review. Oncol Rep. 2003; Nov-Dec. 10(6):1663–1682. PMID: 14534679.
Article5. Domercq M, Matute C. Neuroprotection by tetracyclines. Trends Pharmacol Sci. 2004; 12. 25(12):609–612. PMID: 15530637.
Article6. Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000; 7. 6(7):797–801. PMID: 10888929.
Article7. Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004; 5. 07. 279(19):19948–19954. PMID: 15004018.
Article8. Corbacella E, Lanzoni I, Ding D, Previati M, Salvi R. Minocycline attenuates gentamicin induced hair cell loss in neonatal cochlear cultures. Hear Res. 2004; 11. 197(1-2):11–18. PMID: 15504599.
Article9. Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004; 6. 01. 18(11):1272–1282. PMID: 15145826.
Article10. Xu Y, Huang S, Liu ZG, Han J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J Biol Chem. 2006; 3. 31. 281(13):8788–8795. PMID: 16446354.
Article11. Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998; 12. 22. 95(26):15769–15774. PMID: 9861045.
Article12. Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001; 12. 04. 98(25):14669–14674. PMID: 11724929.
Article13. Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001; 4. 15. 21(8):2580–2588. PMID: 11306611.
Article14. Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002; 6. 125(Pt 6):1297–1308. PMID: 12023318.15. Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002; 5. 02. 417(6884):74–78. PMID: 11986668.
Article16. Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci U S A. 2003; 9. 02. 100(18):10483–10487. PMID: 12930891.
Article17. Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006; 6. 20. 103(25):9685–9690. PMID: 16769901.
Article18. Alano CC, Swanson RA. Players in the PARP-1 cell-death pathway: JNK1 joins the cast. Trends Biochem Sci. 2006; 6. 31(6):309–311. PMID: 16679020.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphologic Study of Effect of Fosfomycin and Pentoxifylline on Cisplatin Induced Ototoxicity in Guinea Pig
- Protective Effect of Procaine Hydrochloride on Cisplatin Induced Ototoxicity in Guinea Pig
- Preventive Effect of Fosfomycin on Cisplatin Induced Ototoxicity in Cochlea of Guinea Pig
- Is Hyperbaric Oxygen Therapy Effective in Cisplatin-Induced Ototoxicity in Rats?
- Cisplatin-Induced Ototoxicity: Updates on Potential Molecular Mechanism