J Korean Med Sci.
2013 Jun;28(6):833-839. 10.3346/jkms.2013.28.6.833.
Inhibitory Effect of Melanoma Differentiation Associated Gene-7/Interleukin-24 on Invasion In Vitro of Human Melanoma Cancer Cells
- Affiliations
-
- 1Department of Dermatology, General Hospital of PLA, Beijing, China. hengjinli_hjl@yahoo.com.cn
- 2Department of Oncology, Navy Headquarters Clinics, Beijing, China.
- 3Department of Oncology, General Hospital of PLA, Beijing, China.
- KMID: 2158029
- DOI: http://doi.org/10.3346/jkms.2013.28.6.833
Abstract
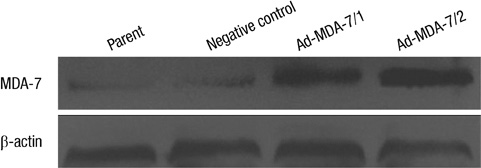
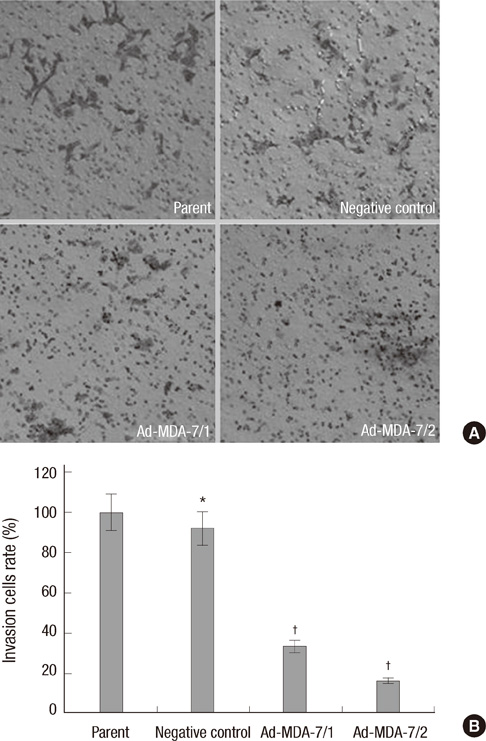
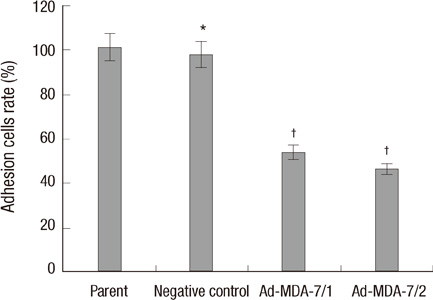
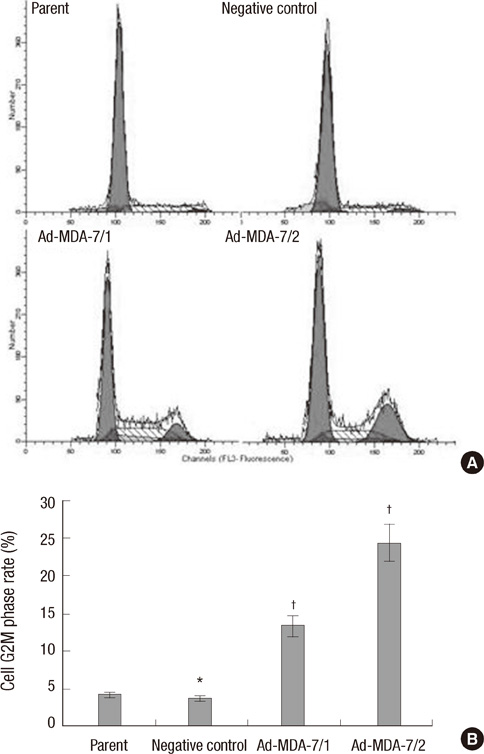
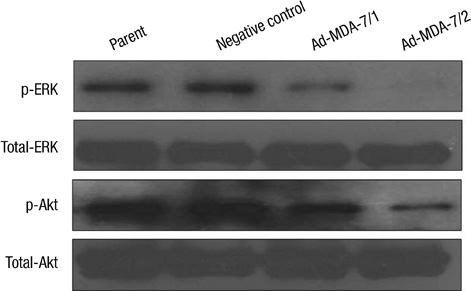
- The acquisition of metastasis potential is a critical point for malignant tumors. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24) is a potential tumor suppress gene and frequently down-regulated in malignant tumors. It has been implicated that overexpression of MDA-7 led to proliferation inhibition in many types of human tumor. Invasion is an important process which is potential to promote tumor metastasis. However, the role and potential molecular mechanism of mda-7/IL-24 to inhibit the invasion of human melanoma cancer is not fully clear. In this report, we identified a solid role for mda-7/IL-24 in invasion inhibition of human melanoma cancer LiBr cells, including decreasing of adhesion and invasion in vitro, blocking cell cycle, down-regulating the expression of ICAM-1, MMP-2/9, CDK1, the phosphorylation of ERK and Akt, NF-kappaB and AP-1 transcription activity. Meanwhile, there was an increased expression of PTEN in mda-7/IL-24 over-expression LiBr cells. Our results demonstrated that mda-7/IL-24 is a potential invasion suppress gene, which inhibits the invasion of LiBr cells by the down-regulation of ICAM-1, MMP-2/9, PTEN, and CDK1 expression. The molecular pathways involved were the MAPK/ERK, PI3K-Akt, NF-kappaB, and AP-1. These findings suggest that mda-7/IL-24 may be used as a possible therapeutic strategy for human melanoma cancer.
MeSH Terms
-
CDC2 Protein Kinase/genetics/metabolism
Cell Line, Tumor
Cell Movement
Down-Regulation
G2 Phase Cell Cycle Checkpoints
Humans
Intercellular Adhesion Molecule-1/genetics/metabolism
Interleukins/genetics/*metabolism
M Phase Cell Cycle Checkpoints
Matrix Metalloproteinase 2/genetics/metabolism
Matrix Metalloproteinase 9/genetics/metabolism
Melanoma/metabolism/pathology
NF-kappa B/genetics/metabolism
PTEN Phosphohydrolase/genetics/metabolism
Phosphorylation
Proto-Oncogene Proteins c-akt/genetics/metabolism
Transcription Factor AP-1/genetics/metabolism
Up-Regulation
Interleukins
NF-kappa B
Transcription Factor AP-1
Intercellular Adhesion Molecule-1
Proto-Oncogene Proteins c-akt
CDC2 Protein Kinase
PTEN Phosphohydrolase
Matrix Metalloproteinase 2
Matrix Metalloproteinase 9
Figure
Reference
-
1. Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, Bray F. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012. 380:1840–1850.2. Turrisi R, Gunn H, Hultgren B, Warner N, Mallett KA. The style project: feasibility of collaborating with salons for prevention and early detection of skin cancer. Arch Dermatol. 2012. 148:1206–1207.3. Lutzky J. New therapeutic options in the medical management of advanced melanoma. Semin Cutan Med Surg. 2010. 29:249–257.4. Allan C, Smithers BM. Surgery and the management of cutaneous melanoma. Br J Surg. 2013. 100:313–315.5. Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008. 14:5610–5618.6. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010. 363:711–723.7. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011. 364:2507–2516.8. Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995. 11:2477–2486.9. Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, Grant S, Dent P, Curiel DT, Sarkar D, et al. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol Ther. 2009. 8:391–400.10. Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, et al. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007. 224:300–307.11. Wang CJ, Zhang H, Chen K, Zheng JW, Xiao CW, Ji WW, Yu Y, Hu HY, Li Y, Xue XB. Ad.mda-7 (IL-24) selectively induces apoptosis in hepatocellular carcinoma cell lines, suppresses metastasis, and enhances the effect of doxorubicin on xenograft tumors. Oncol Res. 2010. 18:561–574.12. Eager R, Harle L, Nemunaitis J. Ad-MDA-7; INGN 241: a review of preclinical and clinical experience. Expert Opin Biol Ther. 2008. 8:1633–1643.13. Gaud G, Iochmann S, Guillon-Munos A, Brillet B, Petiot S, Seigneuret F, Touzé A, Heuzé-Vourc'h N, Courty Y, Lerondel S, et al. TFPI-2 silencing increases tumour progression and promotes metalloproteinase 1 and 3 induction through tumour-stromal cell interactions. J Cell Mol Med. 2011. 15:196–208.14. Sun B, Zhang S, Ni C, Zhang D, Liu Y, Zhang W, Zhao X, Zhao C, Shi M. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 2005. 14:292–298.15. Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012. 13:13240–13263.16. Lydka M, Bilinska B, Cheng CY, Mruk DD. Tumor necrosis factor α-mediated restructuring of the Sertoli cell barrier in vitro involves matrix metalloprotease 9 (MMP9), membrane-bound intercellular adhesion molecule-1 (ICAM-1) and the actin cytoskeleton. Spermatogenesis. 2012. 2:294–303.17. Parfiniewicz B, Pendzich J, Gruchlik A, Hollek A, Weglarz L. Impact of celecoxib on soluble intercellular adhesion molecule-1 and soluble e-cadherin concentrations in human colon cancer cell line cultures exposed to phytic acid and TNF-alpha. Acta Pol Pharm. 2012. 69:1283–1290.18. Zhang P, Ozdemir T, Chung CY, Robertson GP, Dong C. Sequential binding of αVβ3 and ICAM-1 determines fibrin-mediated melanoma capture and stable adhesion to CD11b/CD18 on neutrophils. J Immunol. 2011. 186:242–254.19. Sun X, Essalmani R, Day R, Khatib AM, Seidah NG, Prat A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia. 2012. 14:1122–1131.20. Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010. 21:77–82.21. Dent P, Yacoub A, Hamed HA, Park MA, Dash R, Bhutia SK, Sarkar D, Wang XY, Gupta P, Emdad L, et al. The development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol Ther. 2010. 128:375–384.22. Bonavida B, Baritaki S. The novel role of Yin Yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-κB/Snail/YY1/RKIP/PTEN Circuitry. Crit Rev Oncog. 2011. 16:211–226.23. Eulitt PJ, Park MA, Hossein H, Cruikshanks N, Yang C, Dmitriev IP, Yacoub A, Curiel DT, Fisher PB, Dent P. Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biol Ther. 2010. 10:1290–1305.24. Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, Dmitriev IP, Lesniak MS, Shah K, Grant S, et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010. 18:1130–1142.25. Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008. 78:356–365.26. Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, Chen LZ, Tan HX, Li W, Bi J, et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol Res. 2009. 39:177–186.27. Hamaï A, Meslin F, Benlalam H, Jalil A, Mehrpour M, Faure F, Lecluse Y, Vielh P, Avril MF, Robert C, et al. ICAM-1 has a critical role in the regulation of metastatic melanoma tumor susceptibility to CTL lysis by interfering with PI3K/AKT pathway. Cancer Res. 2008. 68:9854–9864.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A decrease in the expression of CD63 tetraspanin protein elevates invasive potential of human melanoma cells
- Growth and Characterization of the Uveal Melanoma in Vitro
- A New Endothelial Molecule Involved in Melanoma Cell Binding to Human Dermal Microvascular Endothelial Cells
- Effects of B-16 Melanoma Cells and Mycoplasma pneumoniae on the Induction of IL-1 beta, IL-2, IL-6, IL-10, IL-12, and TNF - alpha from Mouse Astrocytes
- The Inhibitory Effect of Phytoclear-EL1 on Melanogenesis