J Korean Med Sci.
2005 Dec;20(6):1046-1052. 10.3346/jkms.2005.20.6.1046.
Antitumor Activity of TRAIL Recombinant Adenovirus in Human Malignant Glioma Cells
- Affiliations
-
- 1Brain Tumor Research, Dong-A University College of Medicine, Busan, Korea. hjlee@dau.ac.kr
- 2Department of Pathology, Pusan National University College of Medicine, Busan, Korea.
- KMID: 2157760
- DOI: http://doi.org/10.3346/jkms.2005.20.6.1046
Abstract
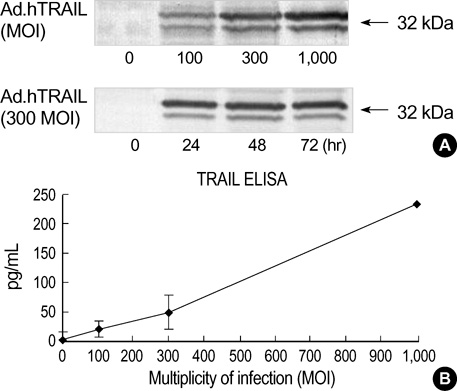
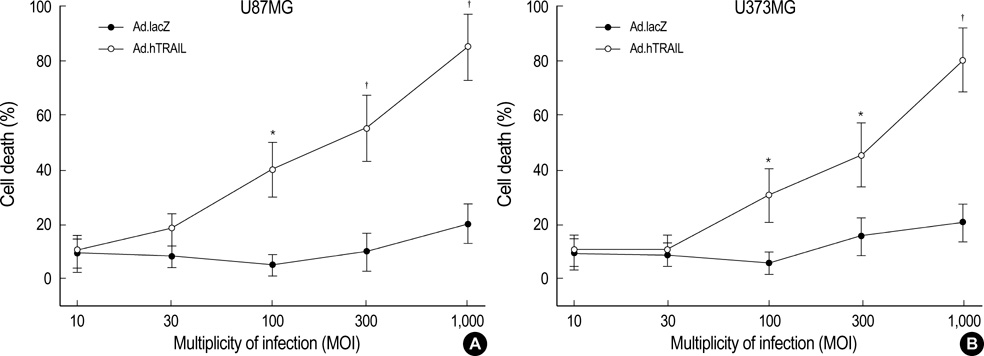
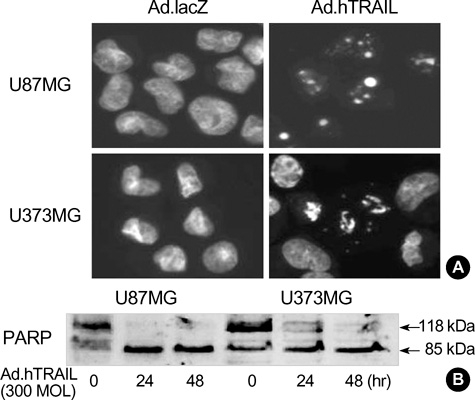
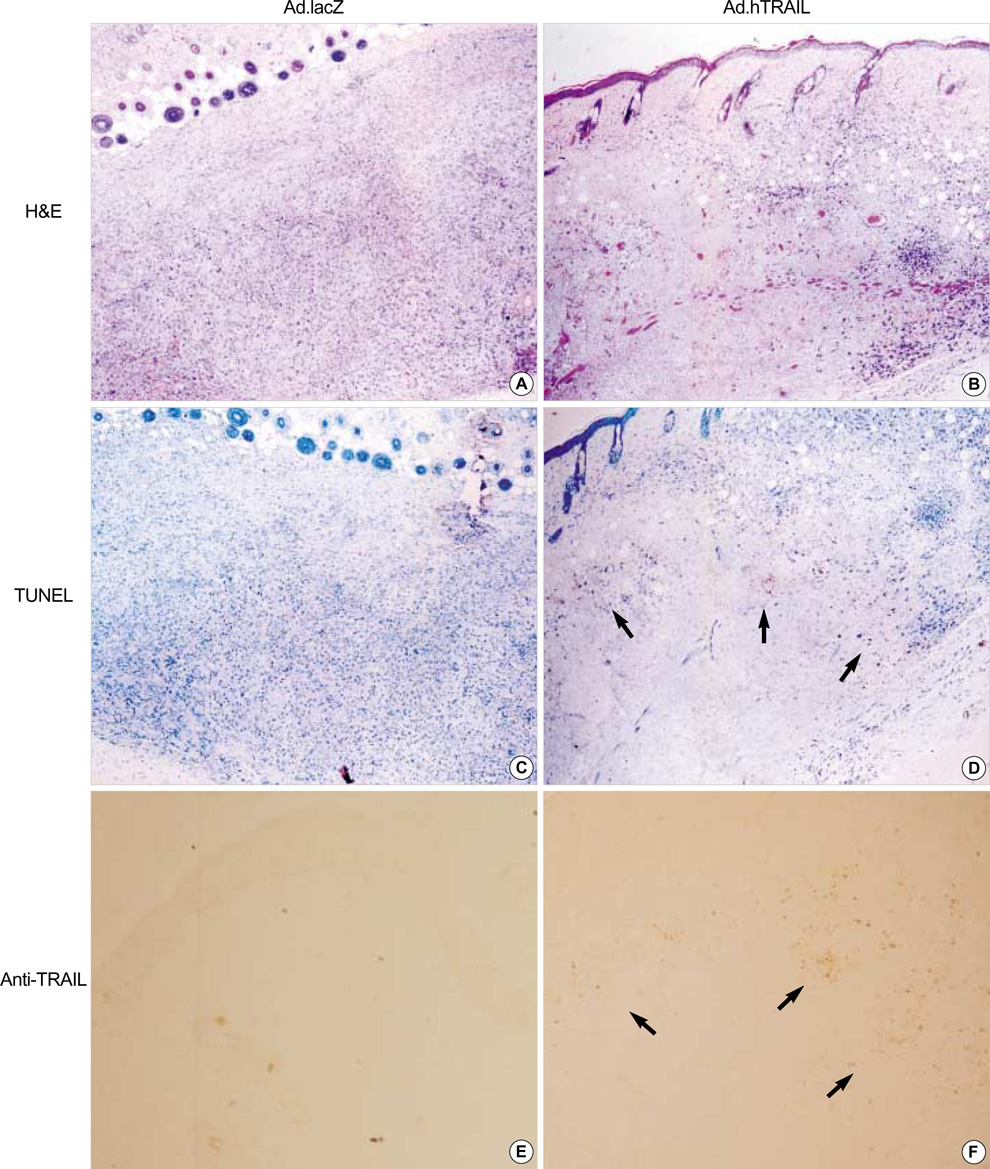
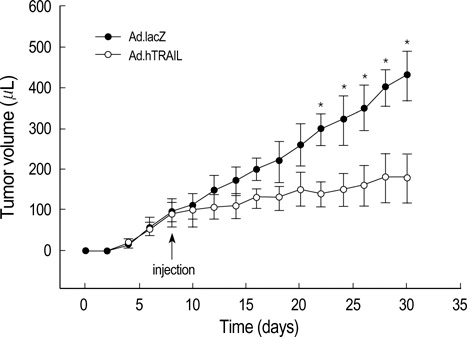
- Tumor necrosis factor-Related Apoptosis-Inducing Ligand (TRAIL) has been reported to specifically kill malignant cells but to be relatively nontoxic to normal cells. One of disadvantages to previous in vivo protocols was the need for large quantities of TRAIL recombinant protein to suppress tumor growth. To evaluate the antitumor activity and therapeutic value of the TRAIL gene, we constructed adenoviral vectors expressing the human TRAIL gene (Ad.hTRAIL) and transferred them into malignant glioma cells in vitro and tumors in vivo, as an alternative to recombinant soluble TRAIL protein. The results show that TRAIL-sensitive glioma cells infected Ad.hTRAIL undergo apoptosis through the production and expression of TRAIL protein. The in vitro transfer elicited apoptosis, as demonstrated by the quantification of viable or apoptotic cells and by the analysis of cleavage of poly (ADP-ribose) polymerase. Furthermore, in vivo administration of Ad.hTRAIL at the site of tumor implantation suppressed the outgrowth of human glioma xenografts in SCID mice. These results further define Ad.hTRAIL as an anti-tumor therapeutic and demonstrate its potential use as an alternative approach to treatment for malignant glioma.
Keyword
MeSH Terms
-
Adenoviridae/*genetics
Animals
Apoptosis
Apoptosis Regulatory Proteins/*genetics
Cell Line, Tumor
Gene Expression
Gene Therapy/*methods
Glioma/pathology/*therapy
Humans
Membrane Glycoproteins/*genetics
Mice
Mice, SCID
Neoplasm Transplantation
Research Support, Non-U.S. Gov't
Transplantation, Heterologous
Tumor Necrosis Factor-alpha/*genetics
Figure
Reference
-
1. Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995. 3:673–682.
Article2. Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 Ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996. 271:12687–12690.
Article3. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999. 104:155–162.
Article4. Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-relatel apoptosis-inducing ligand in vivo. Nat Med. 1999. 5:157–163.5. Gliniak B, Le T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 1999. 59:6153–6158.6. Roth W, Isenmann S, Naumann U, Kugler S, Bahr M, Dichgans J, Ashkenazi A, Weller M. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999. 265:479–483.
Article7. Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000. 97:1754–1759.
Article8. Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997. 276:111–113.
Article9. Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997. 277:818–821.
Article10. Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997. 16:5386–5397.
Article11. MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997. 272:25417–25420.
Article12. Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997. 186:1165–1170.
Article13. Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NFκB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997. 7:813–820.
Article14. Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997. 7:1003–1006.
Article15. Pan G, Ni J, Yu G, Wei YF, Dixit VM. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signaling. FEBS Lett. 1998. 424:41–45.16. Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS, Linet MS. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999. 91:1382–1390.17. Rieger J, Naumann U, Glaser T, Ashkenazi A, Weller M. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 1998. 427:124–128.
Article18. Wu M, Das A, Tan Y, Zhu C, Cui T, Wong MC. Induction of apoptosis in glioma cell lines by TRAIL/Apo-2L. J Neurosci Res. 2000. 61:464–470.
Article19. Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000. 60:847–853.20. Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999. 162:2639–2647.21. Kobayashi K, Oka K, Forte T, Ishida B, Teng B, Ishimura-Oka K, Nakamuta M, Chan L. Reversal of hypercholesterolemia in low density lipoprotein receptor knockout mice by adenovirus-mediated gene transfer of the very low density lipoprotein receptor. J Biol Chem. 1996. 271:6852–6860.
Article22. McGrory WJ, Bautista DS, Graham FL. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988. 163:614–617.
Article23. Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977. 36:59–74.
Article24. Teng B, Blumenthal S, Forte T, Navaratnam N, Scott J, Gotto AM Jr, Chan L. Adenovirus-mediated gene transfer of rat apolipoprotein B mRNA-editing protein in mice virtually eliminates apolipoprotein B-100 and normal low density lipoprotein production. J Biol Chem. 1994. 269:29395–29404.
Article25. Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-κB pathway. Immunity. 1997. 7:821–830.
Article26. Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity. 1997. 7:831–836.
Article27. Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998. 161:2833–2840.28. Weller M, Kleihues P, Dichgans J, Ohgaki H. CD95 ligand: lethal weapon against malignant glioma? Brain Pathol. 1998. 8:285–293.
Article29. Naumann U, Waltereit R, Schulz JB, Weller M. Adenoviral (full-length) Apo2L/TRAIL gene transfer is an ineffective treatment strategy for malignant glioma. J Neurooncol. 2003. 61:7–15.30. Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O'connell M, Kelley RF, Ashkenazi A, de Vos AM. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999. 4:563–571.31. Griffith TS, Broghammer EL. Suppression of tumor growth following intralesional therapy with TRAIL recombinant adenovirus. Mol Ther. 2001. 4:257–266.
Article32. Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, Curley SA, Stephens LC, Fang B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001. 61:3330–3338.33. Song JH, Song DK, Pyrzynska B, Petruk KC, Van Meir EG, Hao C. TRAIL triggers apoptosis in human malignant glioma cells through extrinsic and intrinsic pathways. Brain Pathol. 2003. 13:539–553.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory Effects of Toxoplasma Antigen on Proliferation and Invasion of Human Glioma Cells
- Gene Therapy of Brain Tumors:Effects of Adenovirus-mediated Wild Type p53 Gene Transfer in Human Glioma Cells
- Potentials and limitations of adenovirus-p53 gene therapy for brain tumors
- The Combination of TRAIL Treatment and Cancer Cell Selective Expression of TRAIL-Death Receptor DR4 Induces Cell Death in TRAIL-Resistant Cancer Cells
- Adenovirus Vector-mediated Gene Transfer into Human Trabecular Cell