Ann Dermatol.
2009 Nov;21(4):337-344. 10.5021/ad.2009.21.4.337.
Expression of Toll-like Receptor 2 in Cultured Human Keratinocytes: The Effect of Bacterial Antigens, Cytokines and Calcium Concentration
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Kyung Hee University, Seoul, Korea. nikim@khmc.or.kr
- KMID: 2156494
- DOI: http://doi.org/10.5021/ad.2009.21.4.337
Abstract
- BACKGROUND
Toll-like receptors (TLRs) are expressed by human epidermal keratinocytes and are involved in immune responses.
OBJECTIVE
The goal of this was to investigate the expression of TLR2 in response to bacterial antigens, cytokines, and different calcium concentrations.
METHODS
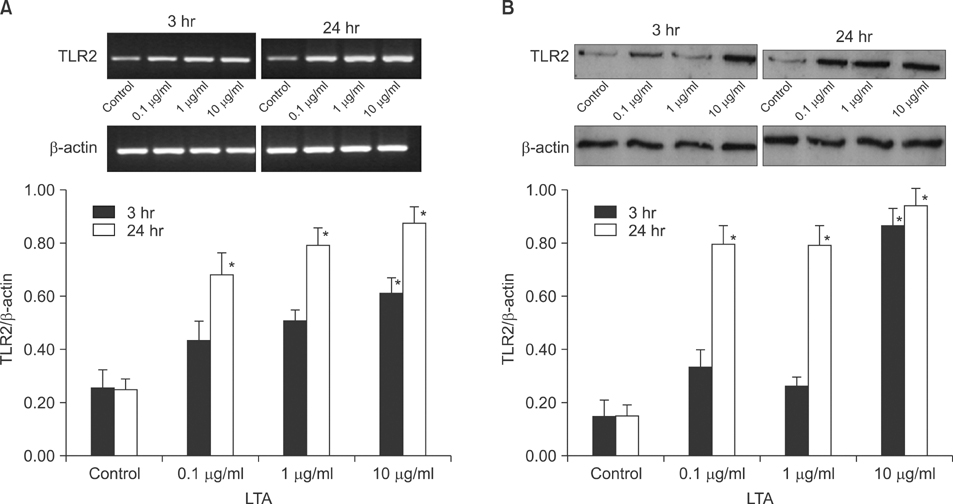
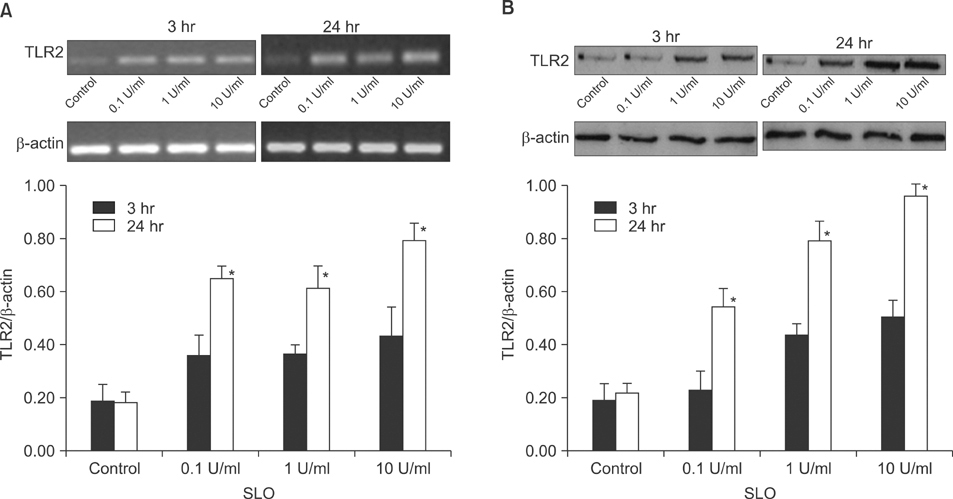
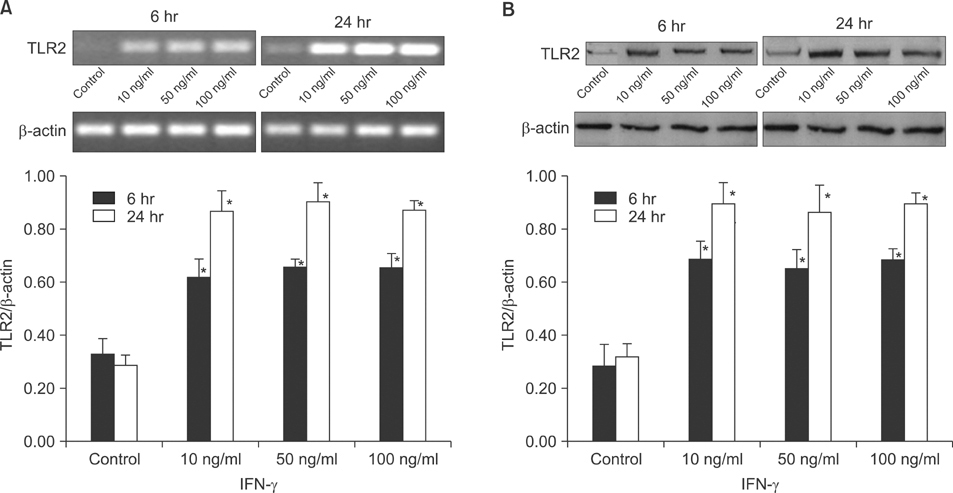
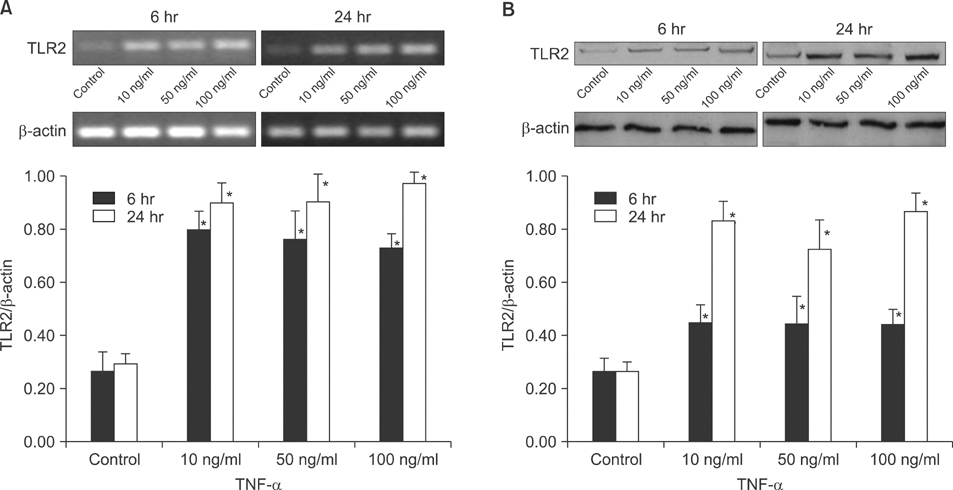
The expression of TLR2 was assessed after stimulation by lipoteichoic acid (LTA) and streptolysin O (SLO). In addition, TLR2 expression was evaluated after treatment with IFN-gamma and TNF-alpha, and different concentrations of calcium. The expression levels of TLR2 mRNA and protein were studied using RT-PCR and Western blot analysis.
RESULTS
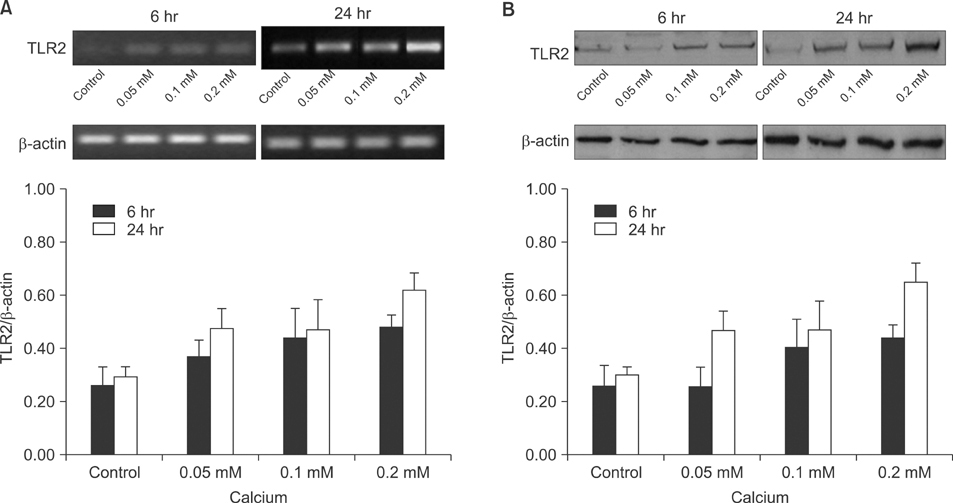
Cultured human epidermal keratinocytes constitutively expressed TLR2 and the expression was stimulated by LTA and SLO; in addition, IFN-gamma and TNF-alpha upregulated TLR2 expression. However, the changes in TLR2 expression associated with the calcium concentrations were insignificant.
CONCLUSION
TLR2 expression increased with the concentration and duration of bacterial pathogens and this increase was amplified by several cytokines, from activated keratinocytes and other cells.
MeSH Terms
-
Antigens, Bacterial
Bacterial Proteins
Blotting, Western
Calcium
Cytokines
Humans
Keratinocytes
Lipopolysaccharides
RNA, Messenger
Streptolysins
Teichoic Acids
Toll-Like Receptor 2
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Antigens, Bacterial
Bacterial Proteins
Calcium
Cytokines
Lipopolysaccharides
RNA, Messenger
Streptolysins
Teichoic Acids
Toll-Like Receptor 2
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996. 86:973–983.
Article2. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998. 95:588–593.3. Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999. 11:13–18.
Article4. O'Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998. 63:650–657.5. Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999. 401:811–815.
Article6. Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999. 163:1–5.7. Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999. 274:33419–33425.
Article8. Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999. 285:732–736.
Article9. Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A. 1999. 96:14459–14463.
Article10. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998. 282:2085–2088.
Article11. Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med. 1999. 189:615–625.
Article12. Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995. 95:55–65.
Article13. Wang B, Ruiz N, Pentland A, Caparon M. Keratinocyte proinflammatory responses to adherent and nonadherent group A streptococci. Infect Immun. 1997. 65:2119–2126.
Article14. Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007. 127:331–341.
Article15. Mempel M, Voelcker V, Kollisch G, Plank C, Rad R, Gerhard M, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003. 121:1389–1396.
Article16. Kang SS, Kauls LS, Gaspari AA. Toll-like receptors: applications to dermatologic disease. J Am Acad Dermatol. 2006. 54:951–983.
Article17. Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001. 291:1544–1547.
Article18. Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002. 109:1351–1359.
Article19. Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003. 148:670–679.
Article20. Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002. 119:424–432.
Article21. Pivarcsi A, Bodai L, Rethi B, Kenderessy-Szabo A, Koreck A, Szell M, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003. 15:721–730.
Article22. Lebre MC, Antons JC, Kalinski P, Schuitemaker JH, van Capel TM, Kapsenberg ML, et al. Double-stranded RNA-exposed human keratinocytes promote Th1 responses by inducing a Type-1 polarized phenotype in dendritic cells: role of keratinocyte-derived tumor necrosis factor alpha, type I interferons, and interleukin-18. J Invest Dermatol. 2003. 120:990–997.23. Park YM, Kwon HJ, Kang YS, Koo JK, Kim MY, Kim HO, et al. Expression of Toll-like receptor 4 on human keratinocytes by lipoteichoic acid. Korean J Dermatol. 2006. 44:15–21.24. Aly R, Maibach HE, Mandel A. Bacterial flora in psoriasis. Br J Dermatol. 1976. 95:603–606.
Article25. Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993. 92:1374–1380.
Article26. McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005. 125:1–8.
Article27. Akashi-Takamura S, Miyake K. Toll-like receptors (TLRs) and immune disorders. J Infect Chemother. 2006. 12:233–240.
Article28. Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol. 2006. 84:333–341.
Article29. Mizel SB, Honko AN, Moors MA, Smith PS, West AP. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol. 2003. 170:6217–6223.
Article30. Dahl MV. Imiquimod: a cytokine inducer. J Am Acad Dermatol. 2002. 47:S205–S208.
Article31. Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003. 171:3977–3982.
Article32. Watt FM, Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982. 295:434–436.
Article33. Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci. 2002. 30:185–194.
Article34. Bernard BA, Asselineau D, Schaffar-Deshayes L, Darmon MY. Abnormal sequence of expression of differentiation markers in psoriatic epidermis: inversion of two steps in the differentiation program? J Invest Dermatol. 1988. 90:801–805.
Article35. Bonifati C, Ameglio F. Cytokines in psoriasis. Int J Dermatol. 1999. 38:241–251.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peptidoglycan Induces the Production of Interleukin-8 via Calcium Signaling in Human Gingival Epithelium
- Calcitriol May Down-Regulate mRNA Over-Expression of Toll-Like Receptor-2 and -4, LL-37 and Proinflammatory Cytokines in Cultured Human Keratinocytes
- Expression of Toll-like Receptor 4 on Human Keratinocytes by Lipoteichoic Acid
- Expression of cytokeratins and involucrin in cultured human keratinocytes
- Epidermal Growth Factor Attenuated the Expression of Inflammatory Cytokines in Human Epidermal Keratinocyte Exposed to Propionibacterium acnes