Cancer Res Treat.
2016 Jan;48(1):345-354. 10.4143/crt.2014.247.
Forkhead Transcription Factor FOXO1 Inhibits Angiogenesis in Gastric Cancer in Relation to SIRT1
- Affiliations
-
- 1Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea. dslanat@snu.ac.kr
- 2Department of Tumour Biology, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pharmacology, Seoul National University College of Medicine, Seoul, Korea.
- 4Ischemic/Hypoxic Disease Institute Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Anatomy, Dankook University School of Medicine, Cheonan, Korea.
- 8Department of Biomedical Sciences, Inha University College of Medicine, Incheon, Korea.
- KMID: 2152293
- DOI: http://doi.org/10.4143/crt.2014.247
Abstract
- PURPOSE
We previously reported that forkhead transcription factors of the O class 1 (FOXO1) expression in gastric cancer (GC) was associated with angiogenesis-related molecules. However, there is little experimental evidence for the direct role of FOXO1 in GC. In the present study, we investigated the effect of FOXO1 on the tumorigenesis and angiogenesis in GC and its relationship with SIRT1.
MATERIALS AND METHODS
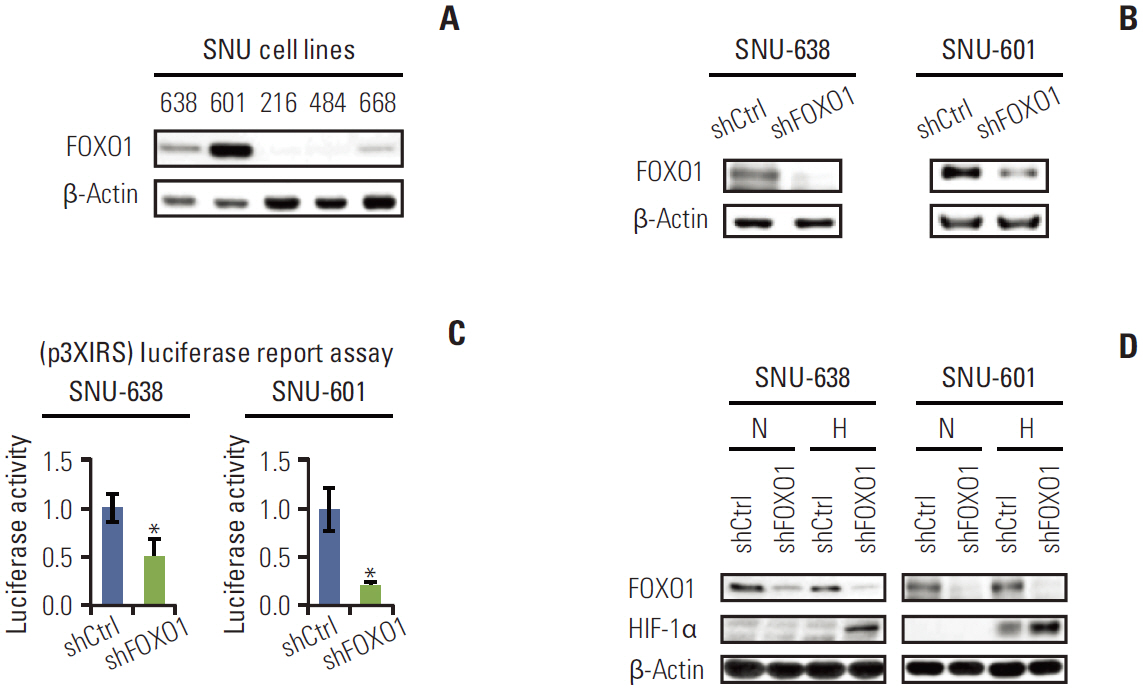
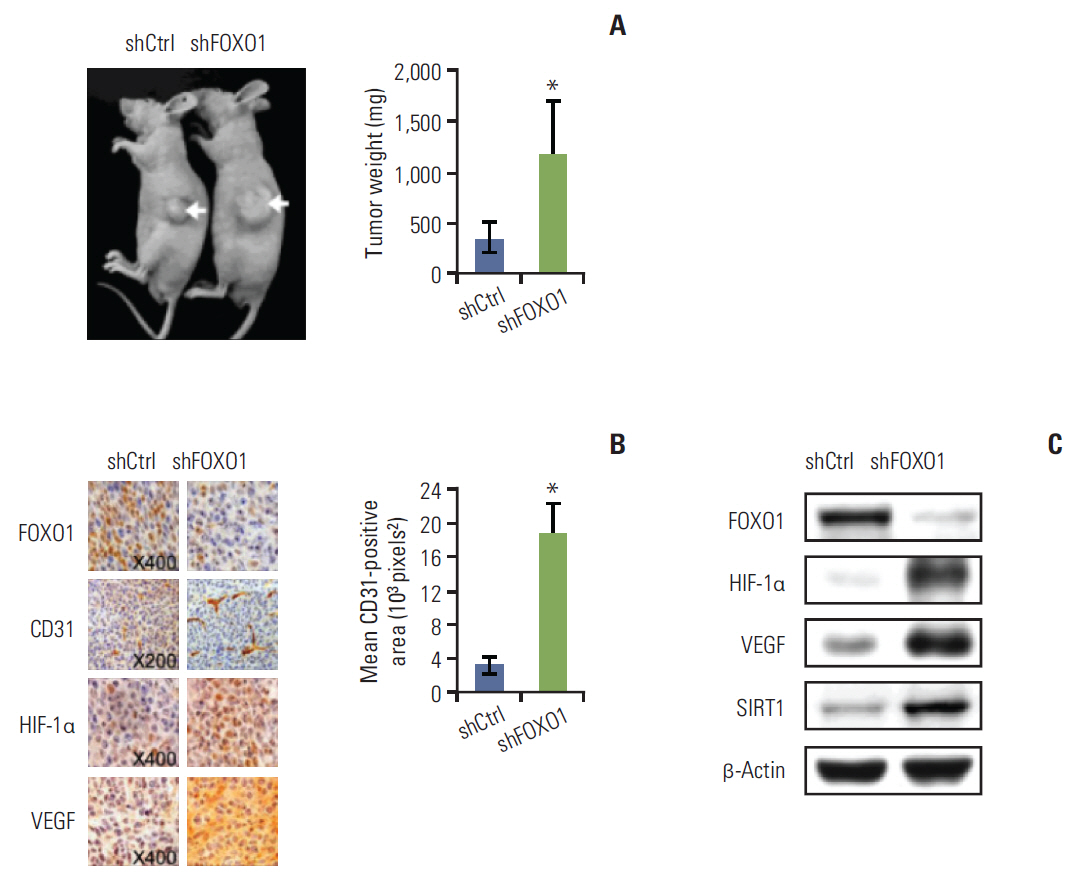
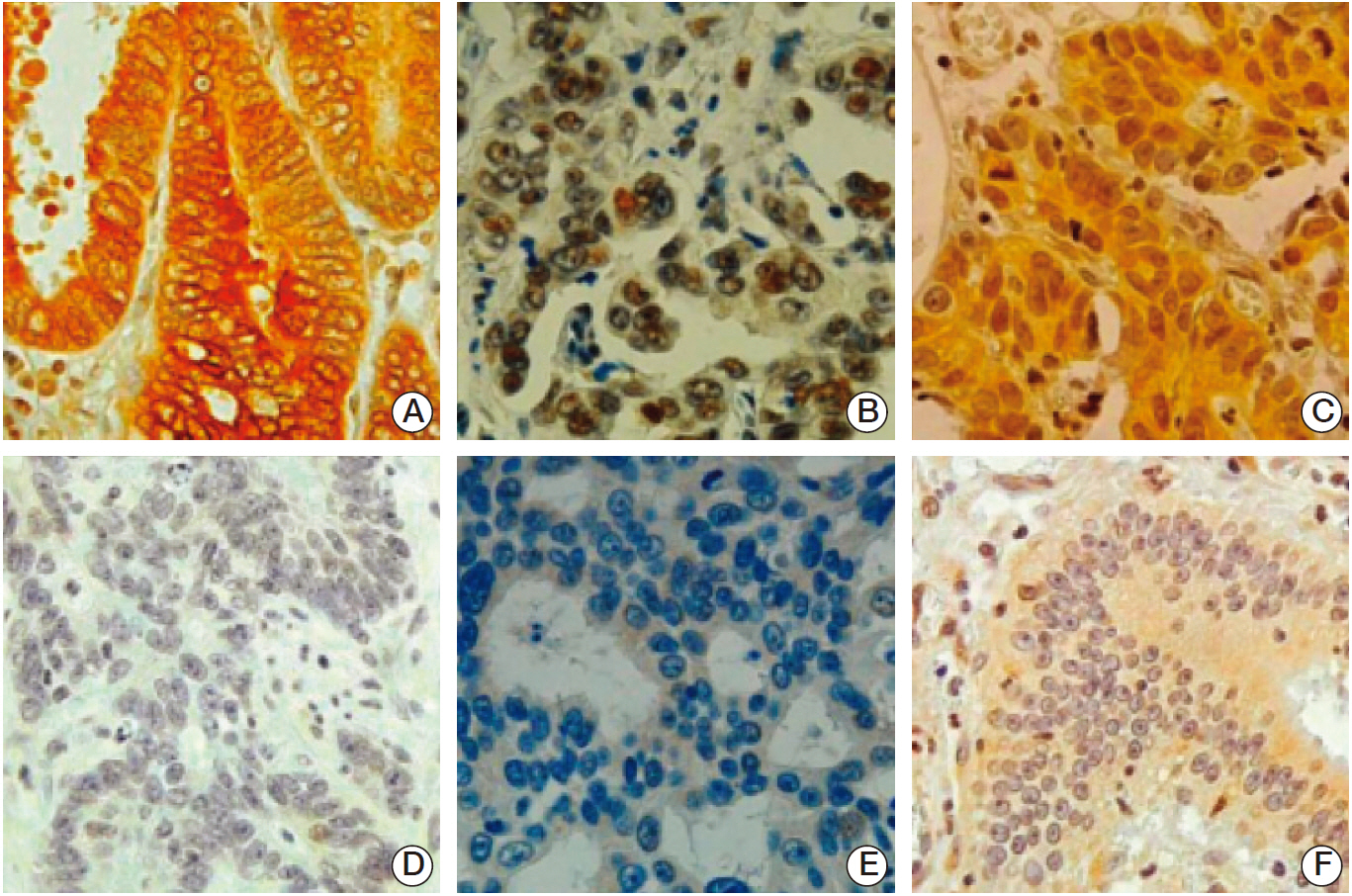
Stable GC cell lines (SNU-638 and SNU-601) infected with a lentivirus containing FOXO1 shRNA were established for animal studies as well as cell culture experiments. We used xenograft tumors in nude mice to evaluate the effect of FOXO1 silencing on tumor growth and angiogenesis. In addition, we examined the association between FOXO1 and SIRT1 by immunohistochemical tissue array analysis of 471 human GC specimens and Western blot analysis of xenografted tumor tissues.
RESULTS
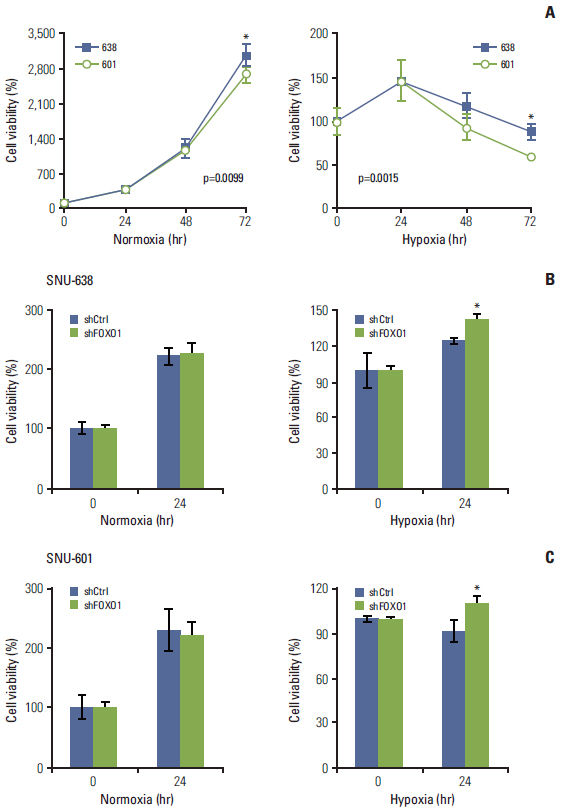
In cell culture, FOXO1 silencing enhanced hypoxia inducible factor-1alpha (HIF-1alpha) expression and GC cell growth under hypoxic conditions, but not under normoxic conditions. The xenograft study showed that FOXO1 downregulation enhanced tumor growth, microvessel areas, HIF-1alpha activation and vascular endothelial growth factor (VEGF) expression. In addition, inactivated FOXO1 expression was associated with SIRT1 expression in human GC tissues and xenograft tumor tissues.
CONCLUSION
Our results indicate that FOXO1 inhibits GC growth and angiogenesis under hypoxic conditions via inactivation of the HIF-1alpha-VEGF pathway, possibly in association with SIRT1. Thus, development of treatment modalities aiming at this pathway might be useful for treating GC.
MeSH Terms
-
Angiogenesis Modulating Agents
Animals
Anoxia
Blotting, Western
Carcinogenesis
Cell Culture Techniques
Cell Line
Down-Regulation
Forkhead Transcription Factors
Heterografts
Humans
Lentivirus
Mice
Mice, Nude
Microvessels
RNA, Small Interfering
Stomach Neoplasms*
Tissue Array Analysis
Transcription Factors*
Vascular Endothelial Growth Factor A
Angiogenesis Modulating Agents
Forkhead Transcription Factors
RNA, Small Interfering
Transcription Factors
Vascular Endothelial Growth Factor A
Figure
Reference
-
References
1. Zhang Y, Gan B, Liu D, Paik JH. FoxO family members in cancer. Cancer Biol Ther. 2011; 12:253–9.
Article2. Kim SY, Yoon J, Ko YS, Chang MS, Park JW, Lee HE, et al. Constitutive phosphorylation of the FOXO1 transcription factor in gastric cancer cells correlates with microvessel area and the expressions of angiogenesis-related molecules. BMC Cancer. 2011; 11:264.
Article3. Srivastava RK, Unterman TG, Shankar S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol Cell Biochem. 2010; 337:201–12.
Article4. Song NY, Surh YJ. Janus-faced role of SIRT1 in tumorigenesis. Ann N Y Acad Sci. 2012; 1271:10–9.
Article5. Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011; 52:1173–85.6. Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010; 38:864–78.7. Yamamoto H, Watanabe Y, Maehata T, Morita R, Yoshida Y, Oikawa R, et al. An updated review of gastric cancer in the next-generation sequencing era: insights from bench to bedside and vice versa. World J Gastroenterol. 2014; 20:3927–37.8. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014; 14:87–104.
Article9. Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004; 14:408–12.
Article10. Nam SY, Ko YS, Jung J, Yoon J, Kim YH, Choi YJ, et al. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-kappaB promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011; 104:166–74.11. Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999; 274:16741–6.
Article12. Kim WH, Schnaper HW, Nomizu M, Yamada Y, Kleinman HK. Apoptosis in human fibrosarcoma cells is induced by a multimeric synthetic Tyr-Ile-Gly-Ser-Arg (YIGSR)-containing polypeptide from laminin. Cancer Res. 1994; 54:5005–10.13. Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003; 200:39–46.
Article14. Hong SS, Lee H, Kim KW. HIF-1alpha: a valid therapeutic target for tumor therapy. Cancer Res Treat. 2004; 36:343–53.15. Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, et al. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004; 96:946–56.16. Wang Y, Li Z, Zhang H, Jin H, Sun L, Dong H, et al. HIF-1alpha and HIF-2alpha correlate with migration and invasion in gastric cancer. Cancer Biol Ther. 2010; 10:376–82.17. Feng AN, Zhang LH, Fan XS, Huang Q, Ye Q, Wu HY, et al. Expression of SIRT1 in gastric cardiac cancer and its clinicopathologic significance. Int J Surg Pathol. 2011; 19:743–50.
Article18. Gong W, Jiang Y, Wang L, Wei D, Yao J, Huang S, et al. Expression of autocrine motility factor correlates with the angiogenic phenotype of and poor prognosis for human gastric cancer. Clin Cancer Res. 2005; 11:5778–83.
Article19. Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004; 279:34741–9.
Article20. Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007; 28:941–53.
Article21. Laemmle A, Lechleiter A, Roh V, Schwarz C, Portmann S, Furer C, et al. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1alpha protein under hypoxic conditions. PLoS One. 2012; 7:e33433.22. Sun P, Yu H, Zhang WQ, Hu M, Lv R. Lentivirus-mediated siRNA targeting VEGF inhibits gastric cancer growth in vivo. Oncol Rep. 2012; 28:1687–92.
Article23. Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010; 7:95–112.
Article24. Maiese K, Chong ZZ, Shang YC, Hou J. A "FOXO" in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009; 29:395–418.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression and Clinical Implications of Forkhead Trasnscription Factor FKHR (FOXO1) in Human Bladder Cancer
- Forkhead box O-class 1 and Forkhead box G1 as Prognostic Markers for Bladder Cancer
- MiR-542-5p Inhibits Hyperglycemia and Hyperlipoidemia by Targeting FOXO1 in the Liver
- SIRT1 Inhibits p53 but not NF-kappaB Transcriptional Activity during Differentiation of Mouse Embryonic Stem Cells into Embryoid Bodies
- Insulin-dependent suppression of cholesterol 7alpha-hydroxlase is a possible link between glucose and cholesterol metabolisms