J Korean Med Sci.
2009 Jun;24(3):468-473. 10.3346/jkms.2009.24.3.468.
Forkhead box O-class 1 and Forkhead box G1 as Prognostic Markers for Bladder Cancer
- Affiliations
-
- 1Department of Urology, School of Medicine, Kyung Hee University, Seoul, Korea.
- 2Department of Urology, Chungbuk National University College of Medicine, Cheongju, Korea. sjyun@chungbuk.ac.kr
- KMID: 1779165
- DOI: http://doi.org/10.3346/jkms.2009.24.3.468
Abstract
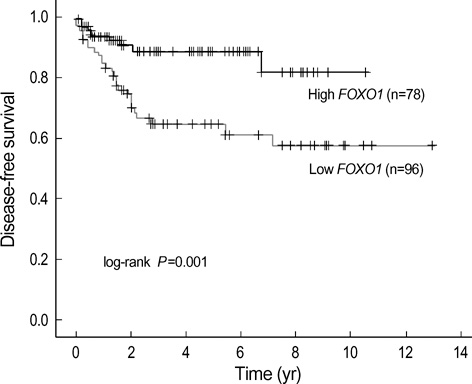
- Forkhead box O-class 1 (FOXO1) is a key regulator of glucose homeostasis, cell-cycle progression, and apoptosis. Its functions are modulated by forkhead box G1 (FOXG1), which acts as a transcriptional repressor with oncogenic potential. Real-time PCR and immunohistochemical staining were performed in 174 primary bladder cancer specimens and 21 normal bladder mucosae to evaluate these genes. FOXO1 and FOXG1 mRNA expression in cancer tissues were higher than in normal mucosae (each P<0.001). FOXO1 mRNA levels were significantly higher in samples of non-progressed patients (P<0.001), but FOXG1 were enhanced in those of progressed patients (P=0.019). On univariate analysis, FOXO1 mRNA expression was significantly associated with grade, stage, recurrence, progression and survival (each P<0.05). On multivariate analysis, increased FOXO1 mRNA expression was associated with both reduced disease progression (odds ratio [OR], 0.367; 95% confidence interval [CI], 0.163-0.826, P=0.015) and enhanced disease-free survival (OR, 3.262; 95% CI, 1.361-7.820, P=0.008). At a median follow-up of 33 months (range 2 to 156), the patients with a high FOXO1 mRNA expression had a significantly prolonged survival (P=0.001). Immunohistochemical findings of FOXO1 were generally concordant with mRNA expression levels. In conclusion, FOXO1 may be a promising marker for predicting progression in human bladder cancers.
Keyword
MeSH Terms
Figure
Reference
-
1. Messing EM. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Urothelial tumors of the bladder. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;2407–2446.2. Evan G, Littlewood T. A matter of life and cell death. Science. 1998. 281:1317–1322.
Article3. Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998. 281:1305–1308.
Article4. Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997. 88:347–354.
Article5. Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002. 277:47928–47937.6. Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003. 100:11285–11290.
Article7. Li J, Thurm H, Chang HW, Iacovoni JS, Vogt PK. Oncogenic transformation induced by the Qin protein is correlated with transcriptional repression. Proc Natl Acad Sci USA. 1997. 94:10885–10888.
Article8. Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004. 117:211–223.
Article9. Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002. 27:352–360.
Article10. Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000. 404:782–787.
Article11. Alvarez B, Martinez AC, Burgering BM, Carrera AC. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature. 2001. 413:744–747.
Article12. Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000. 20:8969–8982.
Article13. Li J, Vogt PK. The retroviral oncogene qin belongs to the transcription factor family that includes the homeotic gene fork head. Proc Natl Acad Sci USA. 1993. 90:4490–4494.
Article14. Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992. 8:957–966.15. Li J, Chang HW, Lai E, Parker EJ, Vogt PK. The oncogene qin codes for a transcriptional repressor. Cancer Res. 1995. 55:5540–5544.16. Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000. 349:629–634.
Article17. Aoki M, Jiang H, Vogt PK. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc Natl Acad Sci USA. 2004. 101:13613–13617.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Expression of Forkhead Box M1 Is Associated with Aggressive Phenotype and Poor Prognosis in Estrogen Receptor-Positive Breast Cancer
- Role of Homeostatic Changes in Salivary Gland Acinar Cells in Primary Sjöögren's Syndrome: A Review
- A Family with Axenfeld-Rieger Syndrome: Report of the Clinical and Genetic Findings
- Prevalence of Foxp3 Positive T Regulatory Cells is Increased during Progression of Cutaneous Squamous Tumors
- Rehmannioside D mitigates disease progression in rats with experimental-induced diminished ovarian reserve via Forkhead Box O1/KLOTHO axis