Korean J Urol.
2007 Feb;48(2):111-119. 10.4111/kju.2007.48.2.111.
Development and Application of Mixed Vaccines in Renal Cell Carcinoma: Combining Autologous Tumor Cells with Dendritic Cells Derived from Autologous or Allogeneic Origin
- Affiliations
-
- 1Department of Urology, College of Medicine, Pochon CHA University, Seongnam, Korea. dsparkmd@ cha.ac.kr
- 2Clinical Research Institute, College of Medicine, Pochon CHA University, Seongnam, Korea.
- KMID: 2139747
- DOI: http://doi.org/10.4111/kju.2007.48.2.111
Abstract
-
PURPOSE: To evaluate the effects of autologous tumor vaccine alone or in combination with dendritic cell vaccines, as a method of stimulating antigen-presenting cells in patients with a locoregionally confined renal cell carcinoma (RCC) or metastatic disease.
MATERIALS AND METHODS
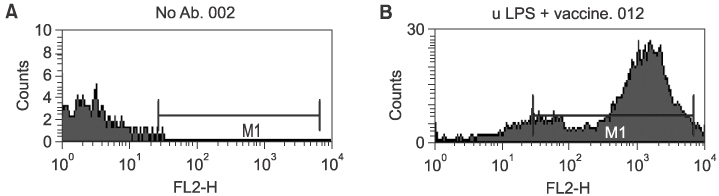
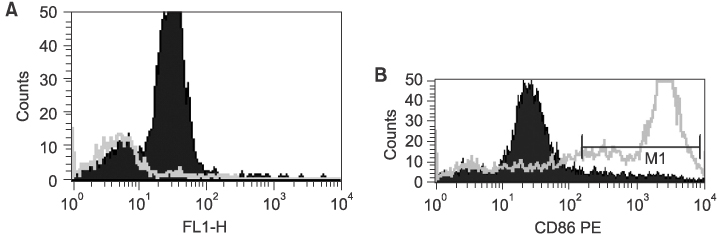
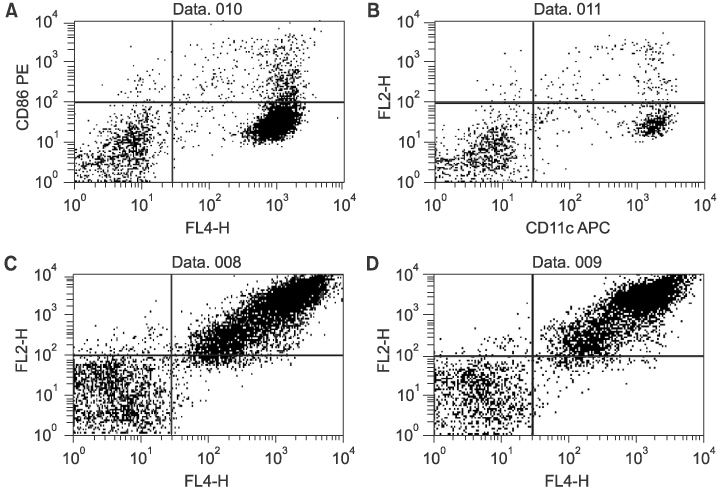
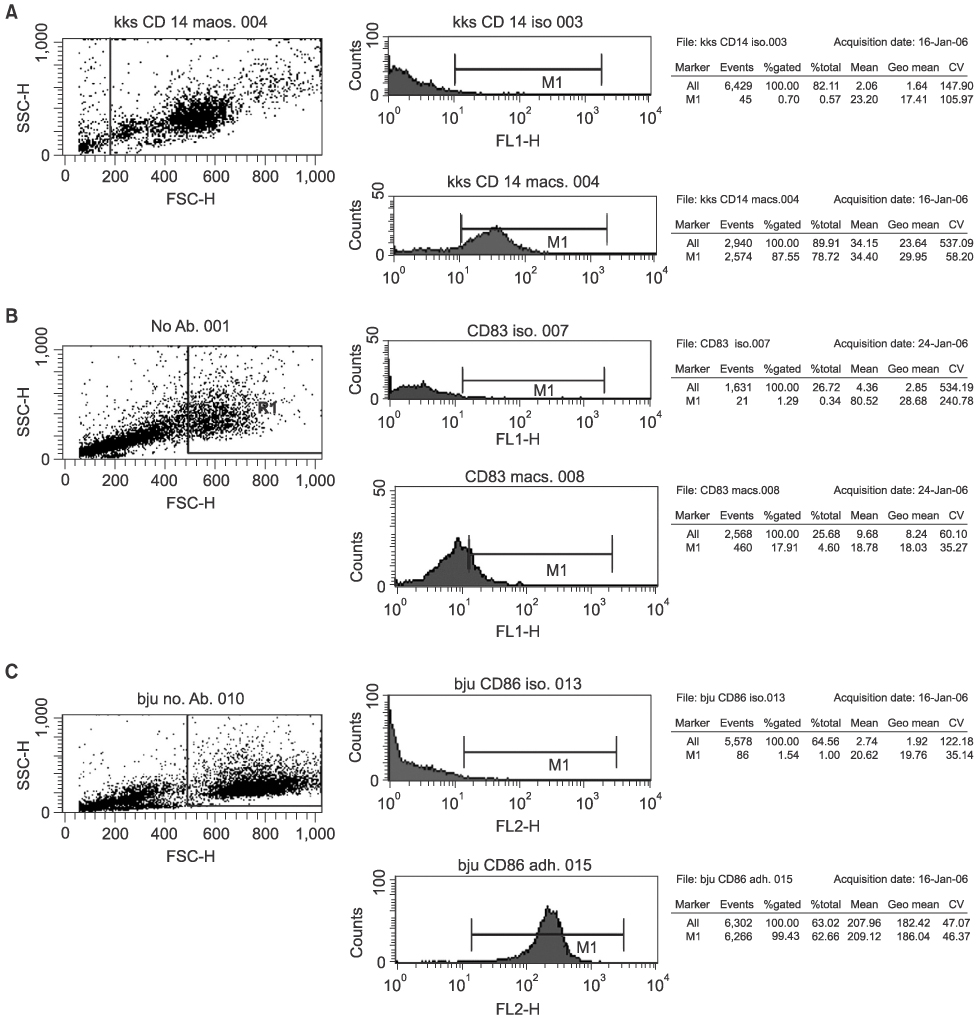
Twenty-seven patients with RCC pathological stages II to IV were treated with autologous tumor cell vaccine, either with or without dendritic cell vaccine. Interleukin 2 (IL-2) based immunotherapy was also applied to the patients with metastatic disease. Immunomagnetic beads were used to isolate CD14+ monocytes from patient or donor in dendritic cell preparations. IL-4 and granulocyte-macrophage colony stimulating factor (GM-CSF) were used for maturation of dendritic cells. Flow cytometry evaluations were performed for dendritic cell maturation and changes in the immunological profiles following our treatment.
RESULTS
Both the isolation of CD14+ monocyte, using Immunomagnetic beads, and the maturation of dendritic cells, using IL-4 and GM-CSF stimulation, were effective. Tumor immunological profiles showed increased CD3 and CD56 populations after treatment. Side effects related with vaccine were minimal and tolerable. Patients were stratified by the purpose for the vaccination; 8 patients for post-nephrectomy adjuvant therapy and 19 for adjuvant immunotherapy of a metastatic disease. All 8 patients in the former showed a disease free state, while only one of the 19 in the latter group remained in complete remission, while 6 showed short-term responses. CONCLISIONS: Autologous RCC vaccine, combined with or without dendritic cell vaccine, might be effective in the suppression of tumor recurrence in locoregionally confined RCC, although a longer follow-up will be required. These vaccines should be further developed to reach their therapeutic purpose in metastatic RCC.
Keyword
MeSH Terms
-
Antigen-Presenting Cells
Carcinoma, Renal Cell*
Colony-Stimulating Factors
Dendritic Cells*
Flow Cytometry
Follow-Up Studies
Granulocyte-Macrophage Colony-Stimulating Factor
Humans
Immunotherapy
Interleukin-2
Interleukin-4
Monocytes
Recurrence
Tissue Donors
Vaccination
Vaccines*
Colony-Stimulating Factors
Granulocyte-Macrophage Colony-Stimulating Factor
Interleukin-2
Interleukin-4
Vaccines
Figure
Cited by 1 articles
-
Immunotherapy for Renal Cell Carcinoma
Dong Soo Park
J Korean Med Assoc. 2008;51(6):569-576. doi: 10.5124/jkma.2008.51.6.569.
Reference
-
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006. 56:106–130.2. Russo P. Renal cell carcinoma: presentation, staging, and surgical treatment. Semin Oncol. 2000. 27:160–176.3. Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006. 295:2516–2524.4. Patel PH, Chaganti RS, Motzer RJ. Targeted therapy for metastatic renal cell carcinoma. Br J Cancer. 2006. 94:614–619.5. Mulders P, Figlin R, deKernion JB, Wiltrout R, Linehan M, Parkinson D, et al. Renal cell carcinoma: recent progress and future directions. Cancer Res. 1997. 57:5189–5195.6. Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep. 2005. 6:7–18.7. Jocham D, Richter A, Hoffmann L, Iwig K, Fahlenkamp D, Zakrzewski G, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renalcell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004. 363:594–599.8. Park DS, Oh DY, Kang MS, An HJ, Lee SJ, Kim NK. Application of autologous tumor vaccine as an adjuvant immunotherapy in the treatment of metastatic renal cell carcinoma. Korean J Urol. 2005. 46:1106–1109.9. Schwaab T, Heaney JA, Schned AR, Harris RD, Cole BF, Noelle RJ, et al. A randomized phase II trial comparing two different sequence combinations of autologous vaccine and human recombinant of autologous vaccine and human recombinant interferon gamma and human recombinant interferon alpha2B therapy in patients with metastatic renal cell carcinoma: clinical outcome and analysis of immunological parameters. J Urol. 2000. 163:1322–1327.10. Brossart P, Wirths S, Brugger W, Kanz L. Dendritic cells in cancer vaccines. Exp Hematol. 2001. 29:1247–1255.11. Flanigan RC. Debulking nephrectomy in metastatic renal cancer. Clin Cancer Res. 2004. 10:6335–6341.12. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994. 179:1109–1118.13. Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003. 15:138–147.14. Holtl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, et al. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002. 8:3369–3376.15. Cheon SH, Chung H, Shin YJ, Kim CS. Culture of dendritic cell from normal peripheral blood monocyte and its anti-tumor immune activity when pulsed by renal cell carcinoma cell line: in vitro study. Korean J Urol. 2002. 43:795–801.16. Avigan D. Dendritic cell-tumor fusion vaccines for renal cell carcinoma. Clin Cancer Res. 2004. 10:6347–6352.17. Michael A, Pandha HS. Renal-cell carcinoma: tumour markers, T-cell epitopes, and potential for new therapies. Lancet Oncol. 2003. 4:215–223.18. Ng CS, Novick AC, Tannenbaum CS, Bukowski RM, Finke JH. Mechanisms of immune evasion by renal cell carcinoma: tumor-induced T-lymphocyte apoptosis and NF-kappaB suppression. Urology. 2002. 59:9–14.19. Finke JH, Zea AH, Stanley J, Longo DL, Mizoguchi H, Tubbs RR, et al. Loss of T-cell receptor zeta chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993. 53:5613–5616.20. Cardi G, Heaney JA, Schned AR, Ernstoff MS. Expression of Fas (APO-1/ CD95) in tumor-infiltrating and peripheral blood lymphocytes in patients with renal cell carcinoma. Cancer Res. 1998. 58:2078–2080.21. Avigan D, Vasir B, Gong J, Borges V, Wu Z, Uhl L, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004. 10:4699–4708.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Separation of Human Epidermal Langerhans Cells by Density Gradient Centrifugation on a Colloidal Silica ( Percoll ) Gradient Method and Autologous , Allogeneic Mixed Skin Cell Leukocyte Culture Reactions
- In vitro Culture of Dendritic Cells and Activation of Peripheral Blood Mononuclear Cells in Patients with Chronic Myelogenous Leukemia
- Immunotherapy for Renal Cell Carcinoma
- Generation of Renal Cell Carcinoma-specific CD4+ /CD8+ T Cells Restricted by an HLA-39 from a RCC Patient Vaccinated with GM-CSF Gene-Transduced Tumor Cells
- Autologous hybrid cell fusion vaccine in a spontaneous intermediate model of breast carcinoma