J Korean Med Assoc.
2008 Jun;51(6):569-576. 10.5124/jkma.2008.51.6.569.
Immunotherapy for Renal Cell Carcinoma
- Affiliations
-
- 1Department of Urology, Pochon Cha University College of Medicine, Korea. dsparkmd@cha.ac.kr
- KMID: 1481929
- DOI: http://doi.org/10.5124/jkma.2008.51.6.569
Abstract
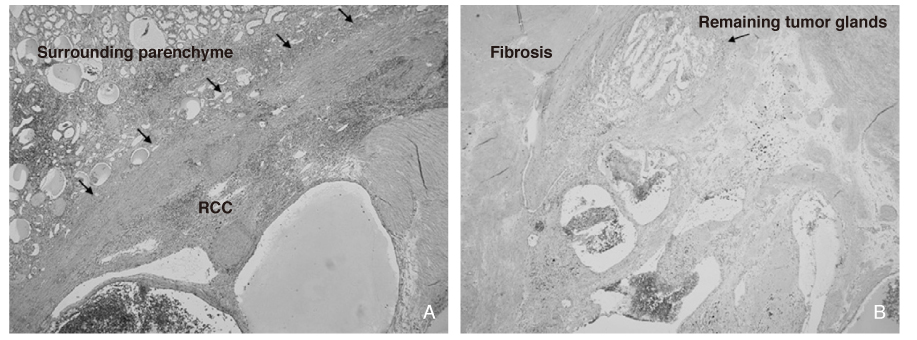
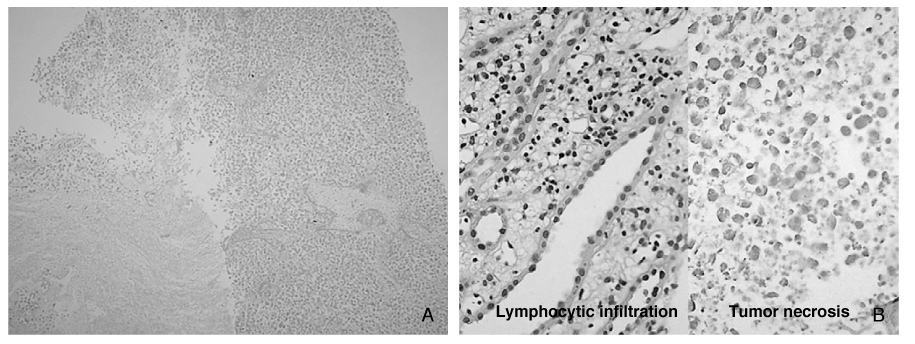
- Since spontaneous regression of metastatic renal cell carcinoma (mRCC) has been reported, immunotherapy for mRCC has been the therapeutic option. The goal of modulating an immune response to the tumor cell with passive and/or active immunotherapy can be achieved through the increasing technological sophistication and the understanding of the immune system. Currently, among the several available cytokines to treat mRCC, high-dose interleukin-2 (IL-2) administration is the only way to obtain complete remission. However, due to the lack of prominent benefit and toxicity of high dose IL-2 therapy, cytokine-based immunotherapy for the treatment of mRCC is threatened by the intriguing molecularly targeted agents, which are still under the trials. Different types of cellular (autologous tumor cell, gene modified tumor cell, dendritic cell) and non-cellular therapeutic vaccines of mRCC have been applied in the clinical setting, and the success of clinical effectiveness in selected population has been reported. Future treatment approaches for mRCC or locally advanced RCC would be directed with combined therapy with immunotherapy and targeted agent. Additionally, molecularly targeted agents and vaccines modulating tumor immunology cascade will be another immunotherapeutic approach for RCC.
Keyword
MeSH Terms
Figure
Reference
-
1. Jenkins GD. Regression of pulmonary metastasis following nephrectomy for hypernephroma: eight year followup. J Urol. 1959. 82:37–40.
Article2. Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA. Ipilimumab (anti-CTL4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007. 30:825–830.
Article3. Parmar S, Plantanias LC. Interferons: mechanisms of action and clinical applications. Curr Opin Oncol. 2005. 15:431–439.
Article4. Rini BI. Is sorafenib plus interferon a2b safe and effective in patients with renal cell carcinoma? Nat Clin Pract Urol. 2008. 5:132–133.
Article5. Costa LJ, Drabkin HA. Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist. 2007. 12:1404–1415.
Article6. Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995. 13:688–696.
Article7. Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000. 6(S):S55–S57.8. Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T, Tursz T. Recombinant human interleukin-2, recombinant human interferon alpha-2a, or both in metastatic renal cell carcinoma. Groupe Francais d'Immunotherapie. N Engl J Med. 1998. 338:1272–1278.
Article9. McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005. 23:133–141.
Article10. Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003. 21:3127–3132.
Article11. Atzpodien J, Korfer A, Palmer PA, Franks CR, Poliwoda H, Kirchner H. Treatment of metastatic renal cell cancer patients with recombinant subcutaneous human interleukin-2 and interferon-alpha. Ann Oncol. 1990. 1:377–378.
Article12. Ravaud A, Negrier S, Cany L, Merrouche Y, Le Guillow M, Blay JY, Clavel M, Gaston R, Oskam R, Philip T. Subcutaneous low-dose recombinant interleukin-2 and alpha interferon in patients with metastatic renal cell carcinoma. Br J Cancer. 1994. 69:1111–1114.
Article13. Huland E, Heinzer H, Huland H, Yung R. Overview of interleukin-2 inhalation therapy. Cancer J Sci Am. 2000. 6:S. S104–S112.14. Atkins M, Regan M, McDermott D, Mier J, Stanbridge E, Youmans A, Febbo P, Upton M, Lechpammer M, Signoretti S. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005. 11:3714–3721.
Article15. Schwaab T, Heaney JA, Schned AR, Harris RD, Cole BF, Noelle RJ, Phillips DM, Stempkowski L, Ernstoff MS. A randomized phase II trial comparing two different sequence combinations of autologous vaccine and human recombinant of autologous vaccine and human recombinant interferon gamma and human recombinant interferon alpha2B therapy in patients with metastatic renal cell carcinoma: clinical outcome and analysis of immunological parameters. J Urol. 2000. 163:1322–1327.
Article16. Jocham D, Richter A, Hoffmann L, Iwig K, Fahlenkamp D, Zakrzewski G, Schmitt E, Dannenberg T, Lehmacher W, von Wietersheim J, Doehn C. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004. 363:594–599.
Article17. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994. 179:1109–1118.
Article18. Brossart P, Wirths S, Brugger W, Kanz L. Dendritic cells in cancer vaccines. Exp Hematol. 2001. 29:1247–1255.
Article19. Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003. 15:138–147.
Article20. Holtl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH Jr, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002. 8:3369–3376.21. Galligioni E, Quaia M, Merlo A, Carbone A, Spada A, Favaro D, Santarosa M, Sacco C, Talamini R. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and bacillus Calmette-Guerin: five-year results of a prospective randomized study. Cancer. 1996. 77:2560–2566.
Article22. Antonia SJ, Seigne J, Diaz J, Muro-Cacho C, Extermann M, Farmelo MJ, Friberg M, Alsarraj M, Mahany JJ, Pow-Sang J, Cantor A, Janssen W. Phase I trial of a B7-1 (CD80) gene modified autologous tumor cell vaccine in combination with systemic interleukin-2 in patients with metastatic renal cell carcinoma. J Urol. 2002. 167:1995–2000.
Article23. Marten A, Renoth S, Heinicke T, Albers P, Pauli A, Mey U, Caspari R, Flieger D, Hanfland P, Von Ruecker A, Eis-Hubinger AM, Muller S, Schwaner I, Lohmann U, Heylmann G, Sauerbruch T, Schmidt-Wolf IG. Allogeneic dendritic cells fused with tumor cells: preclinical results and outcome of a clinical phase I/II trial in patients with metastatic renal cell carcinoma. Hum Gene Ther. 2003. 14:483–494.
Article24. Bleumer I, Tiemessen DM, Oosterwijik-Wakka JC, Voller MC, De Weijer K, Mulders PF, Oosterwijk E. Preliminary analysis of patients with progressive renal cell carcinoma vaccinated with CA9-peptide-pulsed mature dendritic cells. J Immunother. 2007. 30:116–122.
Article25. Avigan DE, Vasir B, George DJ, Oh WK, Atikins MB, Mc-Dermott DF, Kantoff PW, Figlin RA, Vasconcelles MJ, Xu Y, Kufe D, Bukowski RM. Phase I/II study of vaccination with electrofused Allogeneic dendritic cells/autologous tumor-derived cells in patients with stage IV renal cell carcinoma. J Immunother. 2007. 30:749–761.
Article26. Park DS, Cho HJ, Han MY, Lee SJ, Oh DY, Hwang SK. Development and application of mixed vaccines in renal cell carcinoma: combining autologous tumor cells with dendritic cells derived from autologous or allogeneic origin. Korean J Urol. 2007. 48:111–119.
Article27. Uemura H, Fujimoto K, Tanaka M, Yoshikawa M, Hirao Y, Uejima S, Yoshikawa K, Itoh K. A phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinoma. Clin Cancer Res. 2006. 12:1768–1775.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Effect of Angioinfarction with Immunotherapy in Patients with Stage IV Renal Cell Carcinoma
- Novel immunotherapy in metastatic renal cell carcinoma
- Application of Autologous Tumor Vaccine as an Adjuvant Immunotherapy in the Treatment of Metastatic Renal Cell Carcinoma
- Complete Remission of Renal Cell Carcinoma with Metastases to Lung and Bone Following Initial Interferon-alpha Immunotherapy and Adjuvant Nephrectomy
- Metastatic Renal Cell Carcinoma in the Paranasal Sinus: A Case Report and Literature Review