Tuberc Respir Dis.
2015 Jan;78(1):8-17. 10.4046/trd.2015.78.1.8.
Role of AMP-Activated Protein Kinase (AMPK) in Smoking-Induced Lung Inflammation and Emphysema
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine and Clinical Research Center for Chronic Obstructive Airway Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sdlee@amc.seoul.kr
- 2Department of Allergy and Clinical Immunology, Asthma Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2114643
- DOI: http://doi.org/10.4046/trd.2015.78.1.8
Abstract
- BACKGROUND
AMP-activated protein kinase (AMPK) not only functions as an intracellular energy sensor and regulator, but is also a general sensor of oxidative stress. Furthermore, there is recent evidence that it participates in limiting acute inflammatory reactions, apoptosis and cellular senescence. Thus, it may oppose the development of chronic obstructive pulmonary disease.
METHODS
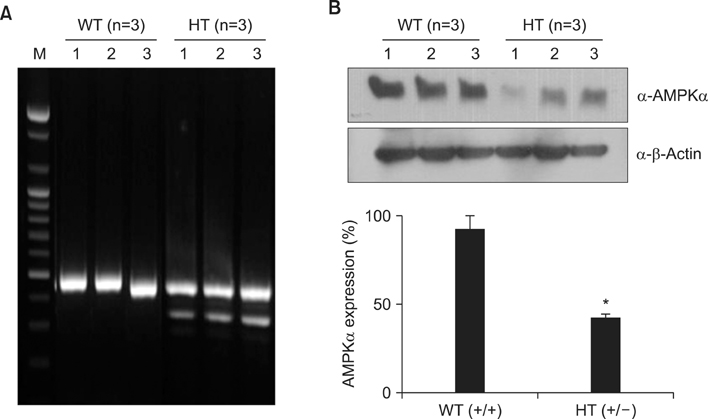
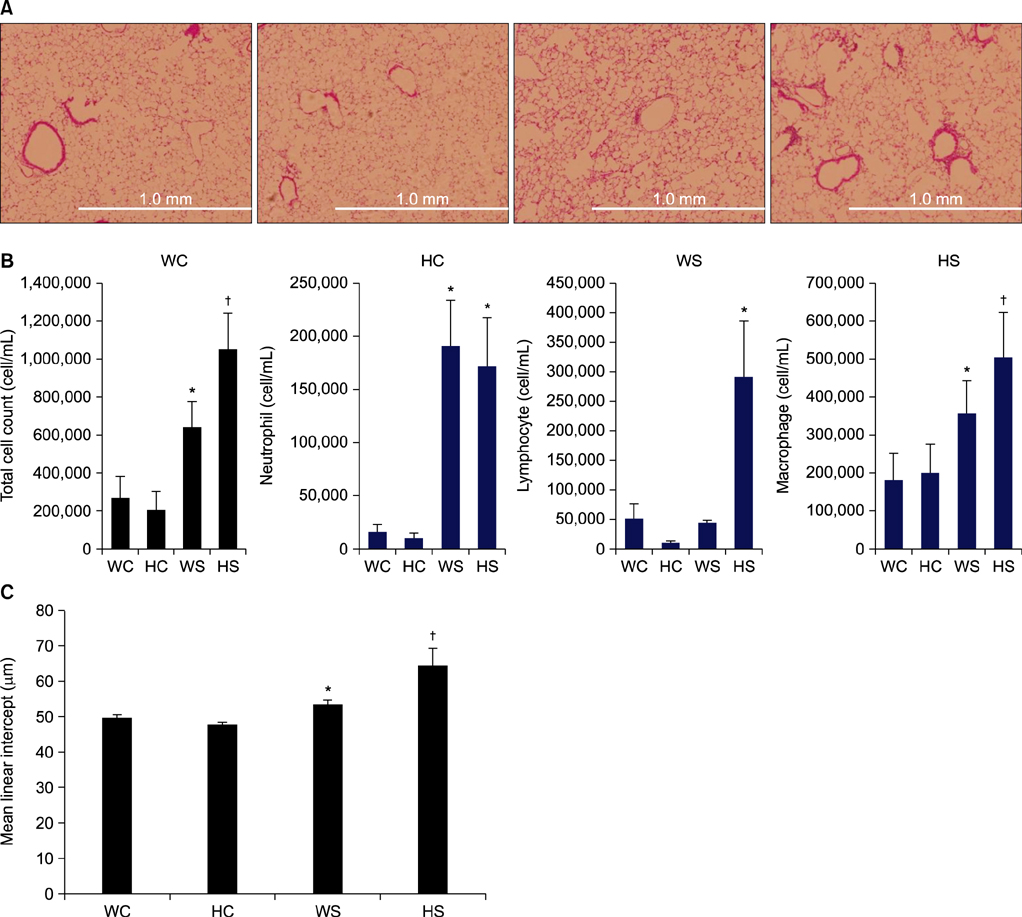
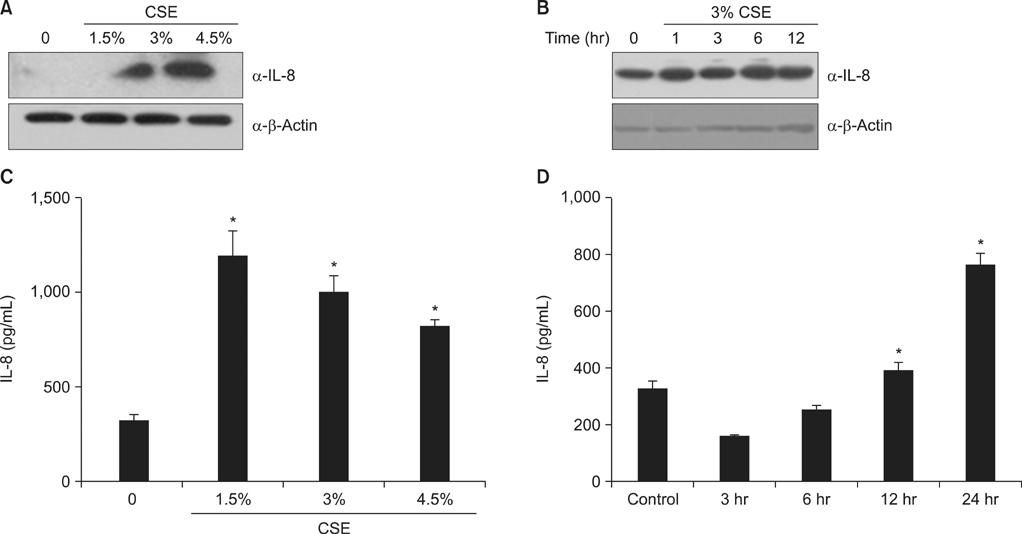
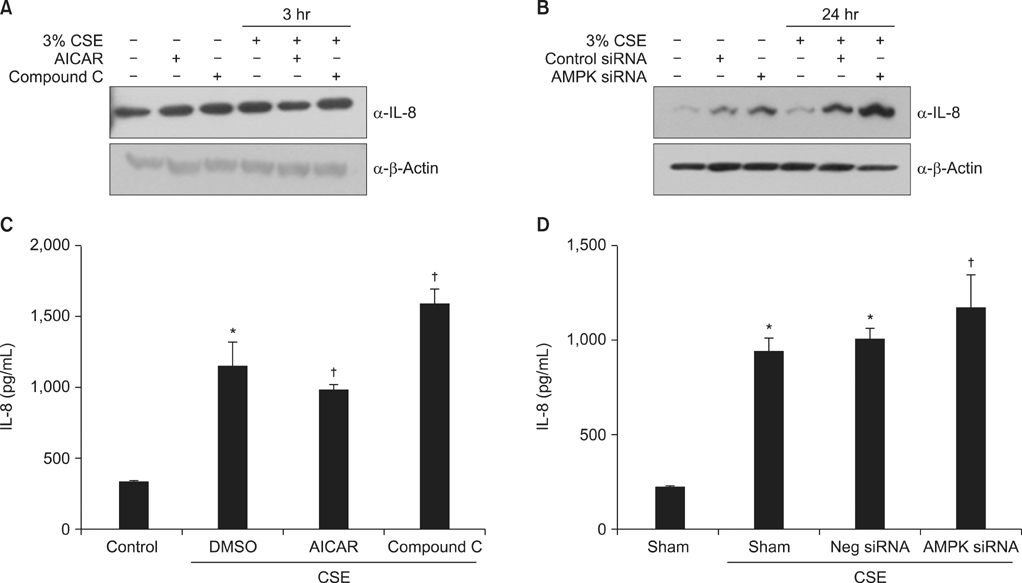
To investigate the role of AMPK in cigarette smoke-induced lung inflammation and emphysema we first compared cigarette smoking and polyinosinic-polycytidylic acid [poly(I:C)]-induced lung inflammation and emphysema in AMPKalpha1-deficient (AMPKalpha1-HT) mice and wild-type mice of the same genetic background. We then investigated the role of AMPK in the induction of interleukin-8 (IL-8) by cigarette smoke extract (CSE) in A549 cells.
RESULTS
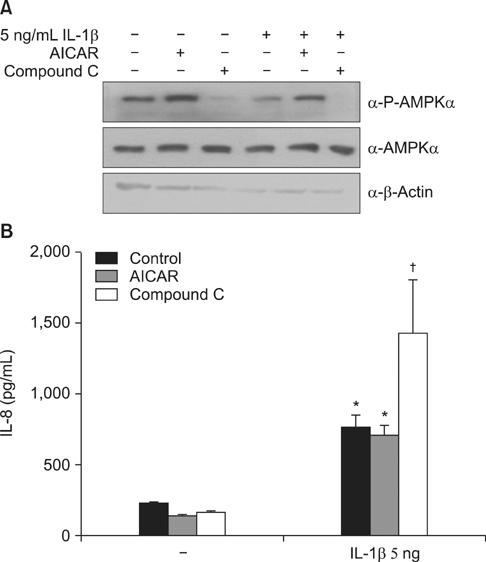
Cigarette smoking and poly(I:C)-induced lung inflammation and emphysema were elevated in AMPKalpha1-HT compared to wild-type mice. CSE increased AMPK activation in a CSE concentration- and time-dependent manner. 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR), an AMPK activator, decreased CSE-induced IL-8 production while Compound C, an AMPK inhibitor, increased it, as did pretreatment with an AMPKalpha1-specific small interfering RNA.
CONCLUSION
AMPKalpha1-deficient mice have increased susceptibility to lung inflammation and emphysema when exposed to cigarette smoke, and AMPK appears to reduce lung inflammation and emphysema by lowering IL-8 production.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Phosphodiesterase 4 Inhibitor Roflumilast Protects against Cigarette Smoke Extract-Induced Mitophagy-Dependent Cell Death in Epithelial Cells
Sun Young Kyung, Yu Jin Kim, Eun Suk Son, Sung Hwan Jeong, Jeong-Woong Park
Tuberc Respir Dis. 2018;81(2):138-147. doi: 10.4046/trd.2017.0115.
Reference
-
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD, [Internet]. Global Initiative for Chronic Obstructive Lung Disease;2013. updated 2013. cited 2014 Dec 1. Available from: http://goldcopd.org.2. Shapiro SD. The pathogenesis of emphysema: the elastase: antielastase hypothesis 30 years later. Proc Assoc Am Physicians. 1995; 107:346–352.3. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977; 1:1645–1648.4. Simmons MS, Connett JE, Nides MA, Lindgren PG, Kleerup EC, Murray RP, et al. Smoking reduction and the rate of decline in FEV(1): results from the Lung Health Study. Eur Respir J. 2005; 25:1011–1017.5. Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5:506–512.6. MacNee W. Oxidative stress and chronic obstructive pulmonary disease. In : Siafakas NM, editor. European Respiratory Monograph, Vol 11. Monograph 38, Management of chronic obstructive pulmonary disease. Wakefield: European Respiratory Society;2006. p. 100–129.7. Henson PM, Cosgrove GP, Vandivier RW. State of the art. Apoptosis and cell homeostasis in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006; 3:512–516.8. Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009; 6:596–601.9. Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005; 352:1967–1976.10. Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009; 360:2445–2454.11. MacNee W, Tuder RM. New paradigms in the pathogenesis of chronic obstructive pulmonary disease I. Proc Am Thorac Soc. 2009; 6:527–531.12. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005; 1:15–25.13. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006; 55:120–127.14. Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007; 5:151–156.15. Tang GJ, Wang HY, Wang JY, Lee CC, Tseng HW, Wu YL, et al. Novel role of AMP-activated protein kinase signaling in cigarette smoke induction of IL-8 in human lung epithelial cells and lung inflammation in mice. Free Radic Biol Med. 2011; 50:1492–1502.16. Nerstedt A, Johansson A, Andersson CX, Cansby E, Smith U, Mahlapuu M. AMP-activated protein kinase inhibits IL-6-stimulated inflammatory response in human liver cells by suppressing phosphorylation of signal transducer and activator of transcription 3 (STAT3). Diabetologia. 2010; 53:2406–2416.17. Myerburg MM, King JD Jr, Oyster NM, Fitch AC, Magill A, Baty CJ, et al. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2010; 42:676–684.18. Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008; 295:L497–L504.19. Hoogendijk AJ, Pinhancos SS, van der Poll T, Wieland CW. AMP-activated protein kinase activation by 5-aminoimidazole-4-carbox-amide-1-beta-D-ribofuranoside (AICAR) reduces lipoteichoic acid-induced lung inflammation. J Biol Chem. 2013; 288:7047–7052.20. Kim TB, Kim SY, Moon KA, Park CS, Jang MK, Yun ES, et al. Five-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside attenuates poly (I:C)-induced airway inflammation in a murine model of asthma. Clin Exp Allergy. 2007; 37:1709–1719.21. Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, Lee KY, et al. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol. 2012; 84:1660–1670.22. Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002; 51:159–167.23. Jia F, Wu C, Chen Z, Lu G. AMP-activated protein kinase inhibits homocysteine-induced dysfunction and apoptosis in endothelial progenitor cells. Cardiovasc Drugs Ther. 2011; 25:21–29.24. Sung JY, Woo CH, Kang YJ, Lee KY, Choi HC. AMPK induces vascular smooth muscle cell senescence via LKB1 dependent pathway. Biochem Biophys Res Commun. 2011; 413:143–148.25. Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012; 11:230–241.26. Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011; 585:986–994.27. Lee JH, Lee DS, Kim EK, Choe KH, Oh YM, Shim TS, et al. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med. 2005; 172:987–993.28. Thurlbeck WM. Measurement of pulmonary emphysema. Am Rev Respir Dis. 1967; 95:752–764.29. Huh JW, Kim SY, Lee JH, Lee JS, Van Ta Q, Kim M, et al. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011; 301:L255–L266.30. Frankenfeld CN, Rosenbaugh MR, Fogarty BA, Lunte SM. Separation and detection of peroxynitrite and its metabolites by capillary electrophoresis with UV detection. J Chromatogr A. 2006; 1111:147–152.31. Yoshida T, Tuder RM. Pathobiology of cigarette smokeinduced chronic obstructive pulmonary disease. Physiol Rev. 2007; 87:1047–1082.32. Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004; 56:515–548.33. Hellermann GR, Nagy SB, Kong X, Lockey RF, Mohapatra SS. Mechanism of cigarette smoke condensate-induced acute inflammatory response in human bronchial epithelial cells. Respir Res. 2002; 3:22.34. Witherden IR, Vanden Bon EJ, Goldstraw P, Ratcliffe C, Pastorino U, Tetley TD. Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastase. Am J Respir Cell Mol Biol. 2004; 30:500–509.35. Masubuchi T, Koyama S, Sato E, Takamizawa A, Kubo K, Sekiguchi M, et al. Smoke extract stimulates lung epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Pathol. 1998; 153:1903–1912.36. Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996; 153:530–534.37. Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N, et al. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 163:349–355.38. Park DW, Jiang S, Tadie JM, Stigler WS, Gao Y, Deshane J, et al. Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol Med. 2013; 19:387–398.39. Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, et al. Activation of the AMPK-FOXO3 pathway reduces fatty acidinduced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009; 58:2246–2257.40. Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008; 57:3222–3230.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation and function of AMPK in physiology and diseases
- Regulation of exercise-stimulated glucose uptake in skeletal muscle
- Humanin suppresses receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via AMP-activated protein kinase activation
- Reserpine treatment activates AMP activated protein kinase (AMPK)
- Effects of AMP-activated Protein Kinase Activating Compounds and Its Mechanism